| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website http://www.currentsurgery.org |
Case Report
Volume 4, Number 2, June 2014, pages 52-54
Cefotetan-Induced Hemolytic Anemia After Laparoscopic Sleeve Gastrectomy
Abdul S. Banguraa, Karen E. Gibbsa, b
aDivision of Minimally Invasive Surgery/Bariatric Surgery, Staten Island University Hospital, Staten Island, New York, USA
bCorresponding Author: Karen E. Gibbs, Staten Island University Hospital, 475 Seaview Avenue, Staten Island, NY 10305, USA
Manuscript accepted for publication June 12, 2014
Short title: Cefotetan-Induced Hemolytic Anemia
doi: https://doi.org/10.14740/jcs210w
| Abstract | ▴Top |
The sleeve gastrectomy is one of several effective bariatric procedures, for morbid obesity. The use of prophylactic perioperative antibiotics in gastrointestinal operations has become standard of care, as it has been shown to reduce surgical site infections. Commonly recommended prophylactic antibiotics include a second or third generation cephalosporin such as cefazolin (Ancef, Kefzol), cefoxitin (Mefoxin) and cefotetan (Cefotan). However, cephalosporins have emerged as a leading cause of immune-mediated hemolytic anemia, with cefotetan and ceftriaxone topping the list. To increase awareness of this rare but potentially fatal phenomenon, we present a case of cefotetan-induced, hemolytic anemia in a patient who had recently undergone a laparoscopic sleeve gastrectomy for morbid obesity.
Keywords: Cefotetan; Hemolytic anemia; Direct antiglobulin test; Coombs test; Morbid obesity; Sleeve gastrectomy
| Introduction | ▴Top |
Drug-induced immune hemolytic anemia (DIIHA) is very rare, and occurs in about one in a million individuals [1]. About 100 drugs have been linked to DIIHA with a positive direct antiglobulin test (DAT), otherwise called the Coombs test. The most common drugs associated with DIIHA in the 1970s were methyldopa and penicillin; currently, cefotetan and ceftriaxone are primarily associated with DIIHA [1-4]. Twelve cephalosporins have been reported to cause DIIHA and five of them (cefotetan, ceftriaxone, ceftizoxime, cefoxitime and cephalothin) have been associated with fatalites [1, 5]. Patients with DIIHA due to cefotetan may have only received one dose of the drug prophylactically with surgery [1]. We present a 41-year-old patient who presented with jaundice 10 days after a laparoscopic sleeve gastrectomy (LSG); the workup indicated immune-mediated hemolytic anemia secondary to cefotetan received prophylactically in the perioperative period. This case is presented in the hope that it can help increase awareness of this serious complication during early postoperative period in bariatric patients.
| Case Report | ▴Top |
A 41-year-old morbidly obese man (501 lbs, 73 inches, BMI 66 kg/m2) with a history of hypertension, obstructive sleep apnea and musculoskeletal pain, underwent an uneventful LSG for the management of morbid obesity. He received perioperative prophylactic antibiotics consisting of 2 g of cefotetan. No blood products were given perioperatively. The patient did well postoperatively and was discharged on postoperative day (POD) 2.
One week later, upon return to the office for the first postoperative visit, the patient was noted to be jaundiced with marked scleral icterus. Upon questioning, he denied noticing the icterus or having acholic stools; he did note that his urine was darker than usual. He admitted feeling some level of fatigue, which he associated to the normal recovery process after surgery. Review of his medications included the standard list of vitamins (multivitamin with iron, B12, and calcium with vitamin D) and hydrocodone bitartrate/acetaminophen (Vicodin)for postoperative pain. His physical exam was otherwise unremarkable. The patient was admitted for workup of his jaundice, and the following initial labs were noted: hemoglobin (Hgb) of 10.3 g/dL, hematocrit (Hct) of 28% with an elevated reticulocyte count of 11.12% (normal 0.5 - 1.5). His preoperative Hgb was 13.8 g/dL and Hct was 39.2%. A complete metabolic panel showed normal liver transaminases and alkaline phosphatase but elevated total bilirubin (Tbili) of 7.8 mg/dL, unconjugated bilirubin of 6.9 mg/dL and lactate dehydrogenase (LDH) of 344 IU/L (normal 160 - 200); conjugated bilirubin was normal at 0.9 mg/dL. These laboratory findings suggested hemolysis as the etiology of the jaundice. A right upper quadrant sonogram and abdominal CT scan showed marked splenomegaly with no other hepatobiliary or abdominal pathology (Fig. 1).
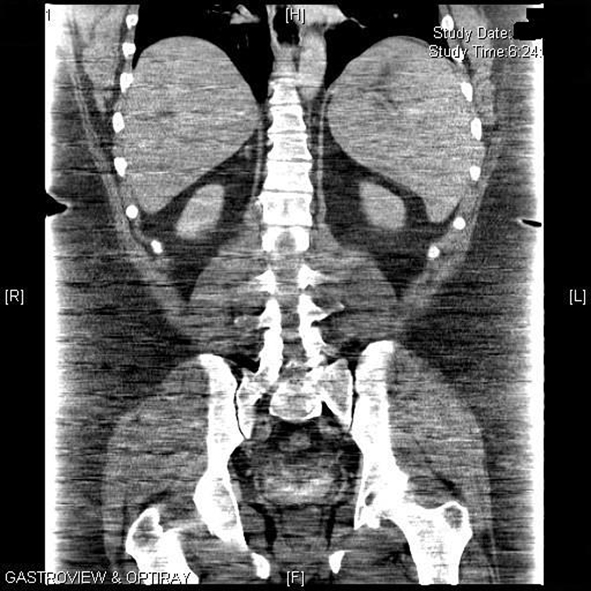 Click for large image | Figure 1. Computed tomography of the abdomen showing marked splenomegaly. |
A hematological consult was obtained and further investigation including a Coombs test (a DAT) was positive. A diagnosis of a DIIHA was entertained and a review of all the medications that patient received in the perioperative period including anesthetic agents, showed cefotetan to be the most likely causative agent based on literature search.
During the initial hospitalization, there was a further drop in his Hgb 7 g/dL/Hct 21% on hospital day 2. Subsequently, the patient became symptomatic with tachycardia and shortness of breath. He was transfused. Steroids were also started once the result of the direct Coombs test was known. On this regimen, his Hgb stabilized and the bilirubin levels gradually normalized; the patient’s symptoms also improved.
After a week of in-hospital care, the patient was discharged home on a tapering dose of prednisone. One month after discharge from the hospital the patient was stable with Hgb 14.0 g/dL and Hct 40.2%.
| Discussion | ▴Top |
Cephalosporins are commonly used antibiotics in the perioperative period. However, we must be aware of their potential associated complications. This case presentation is to increase awareness about the rare but potentially fatal immune-mediated hemolytic anemia associated with these antibiotics. Identification and stopping of the causative agent is the first step in management, preventing fatality from subsequent exposure to the drug.
DIIHA may be a life-threatening complication of antibiotic therapy. The antibiotics most frequently associated with DIIHA are cephalosporins (most commonly cefotetan and ceftriaxone), although over 100 different medications have been directly implicated [1, 2]. Most of the 81 cases of cephalosporin DIIHA reported in the literature have been associated with severe hemolytic anemia; 24% had fatal hemolysis. Less severe cases of hemolytic anemia are likely to have not been diagnosed or reported [1].
Arndt and Garratty reported that cefotetan was the responsible drug in 72% of DIIHA cases referred to their laboratory between 1995 and 2004 [1]. A similar retrospective review of another reference laboratory reports that half of all drug-related antibodies that they investigated were directed against cephalosporins, and of those, 73% were directed against cefotetan [2]. Reports from the American Red Cross Blood Services in Southern California also listed cefotetan as the leading cause (53%) of DIIHA during the period between 1999 and 2008 [3]. The US Food and Drug Administration (FDA) reviewed 85 cases of immune hemolytic anemia associated with cefotetan therapy that had been reported to the FDA and World Health Organization between December 1985 (when cefotetan was approved for use in the United States) and October 1997 [4]. The mean decrease in hemoglobin levels was 6.7 mg/dL (range, 1.6 - 10.3 mg/dL) and the mean final hemoglobin level was 5.2 mg/dL (range, 3 - 9 mg/dL); 55% of patients required transfusion. The mean age of the patients was 52 years and 76% were females. Fifty-nine percent received cefotetan for prophylaxis versus 41% for treatment; in 10% the indication for cefotetan was cesarean section, 13% for other obstetric/gynecology procedures, and 37% for other surgeries. In 30% cefotetan was administered for 1 day versus 70% for more than 1 day; 18% had prior cefotetan administration. The DAT was positive for 50 of 52 patients tested, and cefotetan antibodies were detected in all 30 cases tested. New-onset renal disease was noted in 8.2% of patients and 18% of the hemolytic anemias were fatal.
DIIHA has occurred in patients after 7 - 13 days of therapy with cefotetan. Cefotetan remains RBC-bound in vivo for 16 - 92 days (median of 67 days) [6]; this may explain why hemolytic anemias due to cefotetan persists longer than expected (with other drugs, the hemolytic anemia usually resolves rapidly once the drug is discontinued).
The exact pathophysiology of DIIHA has yet to be fully elucidated. A number of possible mechanisms have been recently described and reviewed [1]. Drug antibodies may be directed at the drug or a combination of drug and erythrocyte membrane protein [1]. Such antibodies may combine with drug on the RBC membrane, either triggering extravascular hemolysis through interaction with macrophages in the reticuloendothelial system or initiating acute intravascular hemolysis via complement activation.
While DIIHA is usually associated with extravascular hemolysis, intravascular hemolysis has been reported with many drugs (e.g., cefotetan and ceftriaxone, especially in children) [1, 7, 8]. All but one of the cases involving piperacillin in which serology or clinical presentations have been described implicate a complement-mediated intravascular process.
The clinical presentation consists of acute and severe anemia, which may be associated with diaphoresis, headache, weakness, paresthesias and tachycardia. Laboratory studies usually show anemia with spherocytes (predominantly extravascular hemolysis) or schistocytes (intravascular hemolysis). Carboxy-hemoglobin levels are commonly elevated during hemolysis, providing an immediate clue to clinicians about the presence of DIIHA [7]. Serum tests typically show a decreased haptoglobin, increased free hemoglobin, increased LDH, total bilirubin and unconjugated bilirubin, and positive DAT for IgG and C3d. Urine will have an increased urobilinogen and free hemoglobin.
Management consists of rapid initiation of supportive care, including intravenous crystalloids to restore intravascular volume and blood transfusion to restore systemic circulation and oxygen carrying capacity. Although corticosteroids and/or intravenous anti-IgG immunoglobulin are often given, there is no supportive evidence that they help suppress immune-mediated hemolysis when drug-dependent antibodies are involved; most reports of successful response to steroids involved simultaneous discontinuation of the drug, which was probably responsible for the resolution of hemolysis. Continuation of the culprit medication may be lethal [5, 8, 9].
Conflict of Interest
There is no conflict of interest related to this manuscript.
| References | ▴Top |
- Arndt PA, Garratty G. The changing spectrum of drug-induced immune hemolytic anemia. Semin Hematol. 2005;42(3):137-144.
doi - Johnson ST, Fueger JT, Gottschall JL. One center's experience: the serology and drugs associated with drug-induced immune hemolytic anemia—a new paradigm. Transfusion. 2007;47(4):697-702.
doi pubmed - Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2009:73-79.
doi pubmed - Viraraghavan R, Chakravarty AG, Soreth J. Cefotetan-induced haemolytic anaemia. A review of 85 cases. Adverse Drug React Toxicol Rev. 2002;21(1-2):101-107.
doi pubmed - Perkins J. Fatal drug-induced immune hemolytic anemia due to cefotetan; A case study. Asian J Transfus Sci. 2008;2(1):20-23.
doi pubmed - Davenport RD, Judd WJ, Dake LR. Persistence of cefotetan on red blood cells. Transfusion. 2004;44(6):849-852.
doi pubmed - Arndt PA, Garratty G, Hill J, Kasper M, Chandrasekaran V. Two cases of immune haemolytic anaemia, associated with anti-piperacillin, detected by the 'immune complex' method. Vox Sang. 2002;83(3):273-278.
doi pubmed - Garratty G, Arndt PA. An update on drug-induced immune hemolytic anemia. Immunohematology. 2007;23(3):105-119.
- Engel RR, Rodkey FL, Krill CE, Jr. Carboxyhemoglobin levels as an index of hemolysis. Pediatrics. 1971;47(4):723-730.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.






