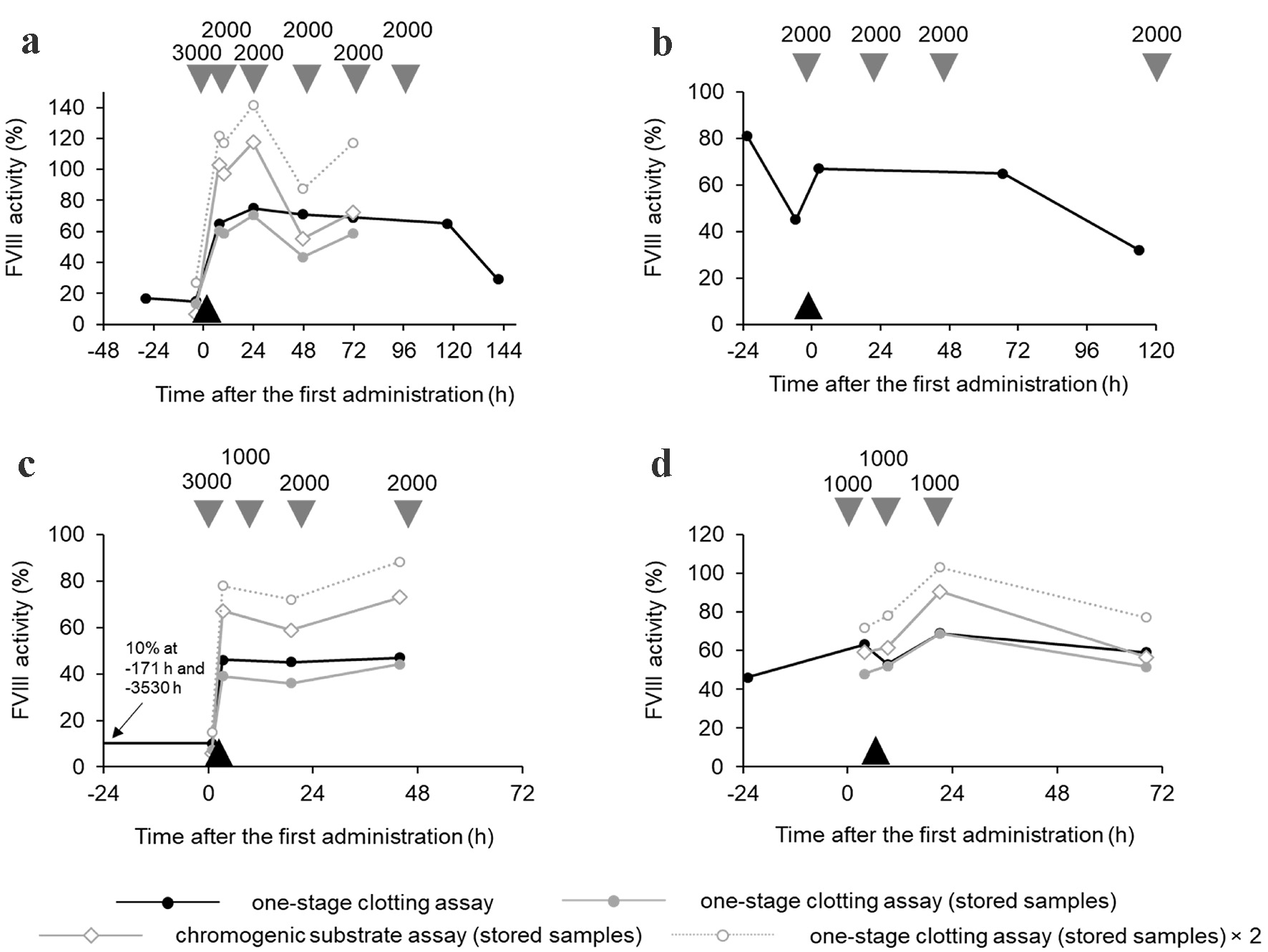
Figure 1. Real-time FVIII activity levels over time for (a) case 1, (b) case 2, (c) case 3, and (d) case 4, measured from the first dose of rVIII-SingleChain. Real-time FVIII activity was measured using the OSA (black circles with solid black lines). Stored plasma samples were used to measure FVIII activity using the OSA (gray circles with solid gray lines) and the CSA (white diamonds with solid gray lines). Converted OSA values (conversion factor = 2) from stored plasma samples are shown (white circles with gray dashed lines). The timing of surgery (black triangles) and the administration of rVIII-SingleChain (gray triangles) are also shown, with the rVIII-SingleChain dose indicated in units. CSA: chromogenic substrate assay; FVIII: factor VIII; OSA: one-stage clotting assay; rVIII: recombinant factor VIII.