| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 12, Number 2, December 2022, pages 50-55
An Unusual Case of Biliary Cystadenoma
Nicholson Branta, Muhammad Darwishb, Houssam Osmanb, Edward Chob, c, Patrick McLarenb, Annie Benzieb, Wareef Kabbanid, Dhiresh Rohan Jeyarajahb, c, e
aTCU/UNTHSC School of Medicine, Fort Worth, TX, USA
bDepartment of Surgery, Methodist Richardson Medical Center, Richardson, TX 75082, USA
cDepartment of Surgery, TCU/UNTHSC School of Medicine, Fort Worth, TX, USA
dDepartment of Pathology, Methodist Health System, Dallas, TX, USA
eCorresponding Author: Dhiresh Rohan Jeyarajah, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX 75082, USA
Manuscript submitted December 28, 2020, accepted January 22, 2021, published online December 30, 2022
Short title: A Case of Biliary Cystadenoma
doi: https://doi.org/10.14740/jcs431
| Abstract | ▴Top |
Biliary cystadenomas (BCAs) are rare, benign, tumors associated with liver parenchyma, and infrequently, the extrahepatic bile ducts. There is potential for malignant transformation, and as such, complete resection is crucial. Removal of the cystic lesion helps differentiate it from other hepatic lesions, such as its malignant counterpart, biliary cystadenocarcinomas (BCACs). We present the case of a 47-year-old female with a recurrence of BCA and a review of the literature. The case outlines an unusual presentation of BCA.
Keywords: Biliary cystadenoma; Liver; Cyst; Ovarian-type stroma
| Introduction | ▴Top |
Biliary cystadenomas (BCAs) are rare tumors with an incidence of less than 5% of all hepatic cystic lesions [1, 2]. They are benign hepatic neoplasms with some malignant potential [3] and are often mistaken for simple hepatic cysts. The majority present asymptomatically and patients often complain of vague abdominal symptoms [4-7]. Current macroscopic criteria and imaging modalities such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), have yet to clearly extrapolate the difference between BCA and biliary cystadenocarcinoma (BCAC) [8]. Complete resection is important to mitigate risk of malignant degeneration, and to help differentiate between BCA and BCAC from other benign cysts. Overall, prognosis and the chance of recurrence are favorable after complete resection.
Here we present the case of a patient with recurrence of BCA in the gallbladder fossa.
| Case Report | ▴Top |
Investigations
A 47-year-old female presented to our clinic with the chief complaint of severe right upper quadrant (RUQ) pain for one month. She complained of cough, vomiting, and headaches during this time. She was 13 years status post laparoscopic cholecystectomy which was not believed to be relevant to her presentation. Physical examination showed the previous surgery’s scars but was otherwise unremarkable.
Diagnosis
A biloma was found on CT and MRI scans. Endoscopic retrograde cholangiopancreatography (ERCP) showed a possible bile leak from the right hepatic duct and a sphincterotomy was performed. A 10-French stent was placed in the common bile duct. Endoscopic US showed no pathology, but drainage continued after stent placement. Labs were within normal limits.
The drain continued to have an output and therefore an MRI and magnetic resonance cholangiopancreatography (MRCP) was obtained. This showed a 1.9-cm mildly complex cystic lesion posterior to the drainage catheter in the gallbladder fossa. There was also mild intrahepatic biliary ductal dilation and borderline extrahepatic biliary ductal dilation with slight wall thickening.
Over the course of the next 8 months the patient continued to have persistent abdominal pain and serous output from the drain despite multiple drain studies proving inconclusive. Nine months status post ERCP an infection was discovered showing cultures that came back positive for Pseudomonas, Serratia, and Acinetobacter in drain fluid. She was started on trimethoprim-sulfamethoxazole (10 days) and levofloxacin (14 days) and her drain was replaced.
The patient returned for her RUQ pain with dyspepsia, nausea, and vomiting. US at that time also noted a large multilocular fluid collection in the region of the gallbladder fossa with septations and debris. Repeat imaging with MRI showed an increase in size to 10.4 × 10.2 × 14.1 cm of the multilocular cystic process involving the right lobe of the liver and gallbladder fossa (Fig. 1). Differential diagnosis at that time included a complex cyst, BCA/cystadenocarcinoma, complex biloma, or a possible lymphangioma. She was seen by surgery at this time. The patient was counseled about the need for surgery for definitive diagnosis.
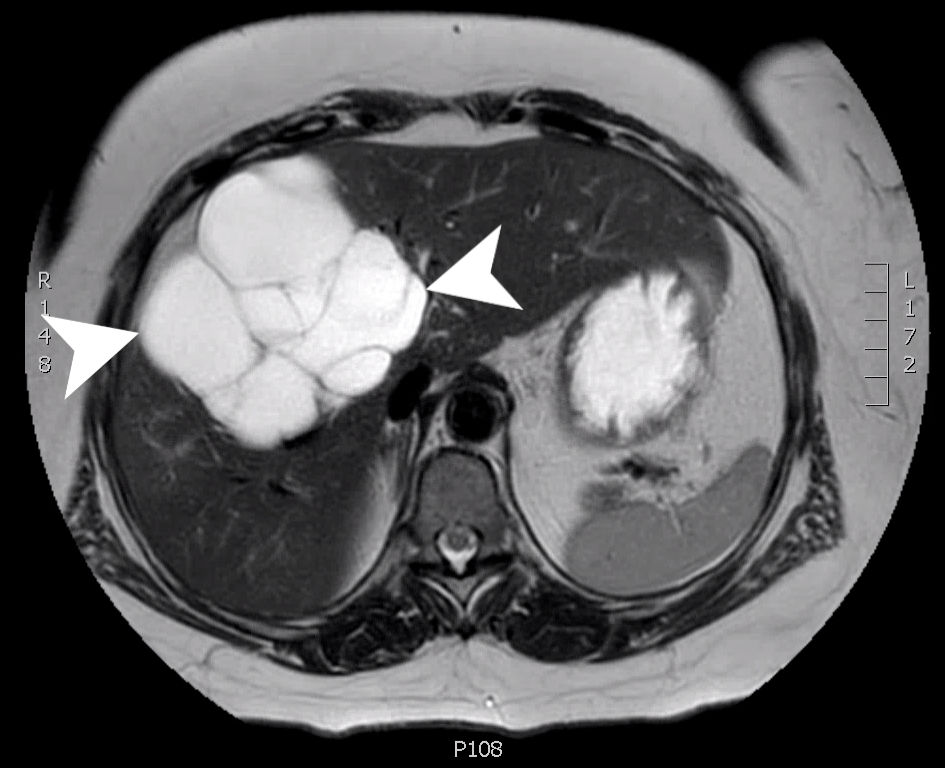 Click for large image | Figure 1. An axial slice from a T2 weighted MRI with gadolinium contrast showing a complex, multilocular cystic mass (arrow heads) measuring 10.4 × 10.2 × 14.1 cm involving segments 8, 5 and 4B. MRI: magnetic resonance imaging. |
Treatment
The patient subsequently underwent an exploratory laparotomy and resection of all visible cyst areas. Intraoperatively, a large cystic lesion measuring 10.4 × 10.2 × 14.1 cm involving segments 8, 5, and 4b was found in the liver. The lesion extended into the porta hepatis and anteriorly over the pancreatic head and descending duodenum. After removal, the initial biopsy was consistent with a BCA. On gross examination, the lesion is cystic with multiple communicating lobules of varying sizes with a smooth and glistening lining. All areas of viable cyst were resected. Microscopic examination revealed a hepatobiliary cystadenoma without atypia or malignancy (Fig. 2).
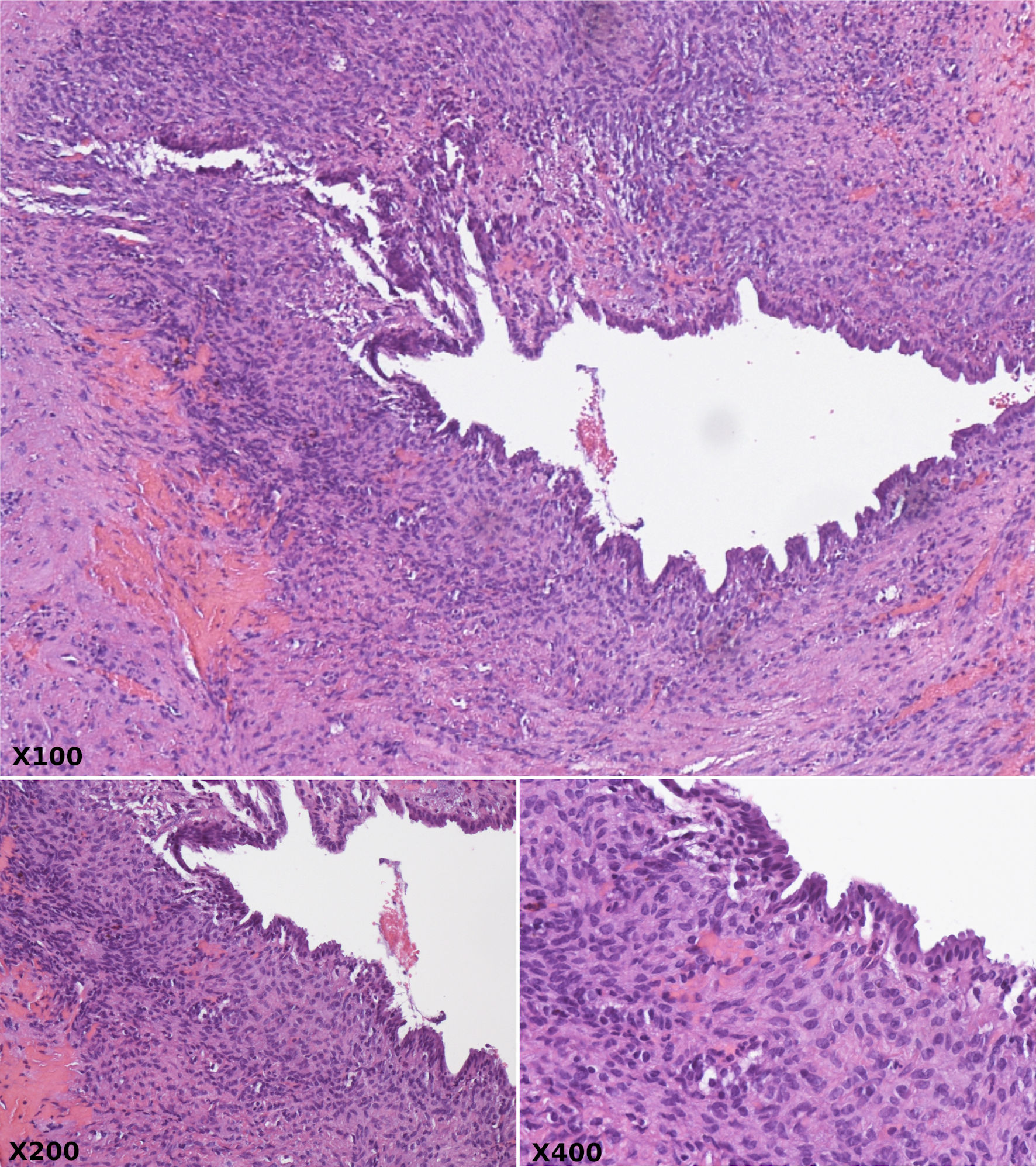 Click for large image | Figure 2. Hematoxylin and eosin stain at × 100, × 200, and × 400 magnification revealing cystically dilated ducts lined by bland biliary columnar epithelium and surrounded by compact ovarian type spindle cell stroma. |
She was followed for routine monitoring; however, an MRI 5 months later showed that there was still a cystic area in the liver bed where the initial resection of the cystadenoma occurred (Fig. 3). At the time it was unclear whether this was residual fluid collection from surgery, or recurrent BCA.
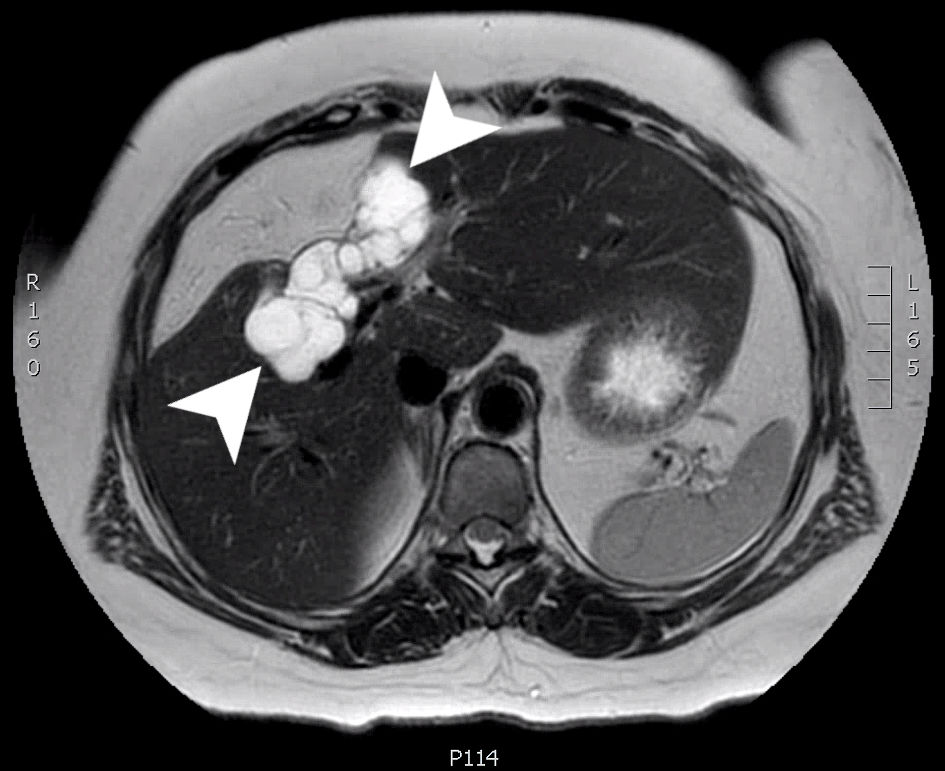 Click for large image | Figure 3. An axial slice from a T2 weighted MRI scan with Eovist (gadoxetate disodium) contrast. A multiloculated cystic mass (arrow heads) is seen involving the right hepatic lobe (segments 4 and 5) measuring 8.0 × 3.2 × 3.2 cm. MRI: magnetic resonance imaging. |
An MRI at 13 months postoperatively showed significant growth of the cyst, now measuring 12 × 9 × 10 cm, (previously 9 × 4 × 7 cm) and some mild intrahepatic biliary dilation (Fig. 4). During that visit, the patient reported pruritus, dark orange urine, and mustard-colored stools 2 weeks prior which had since resolved. The patient was again counseled about the need for surgical removal.
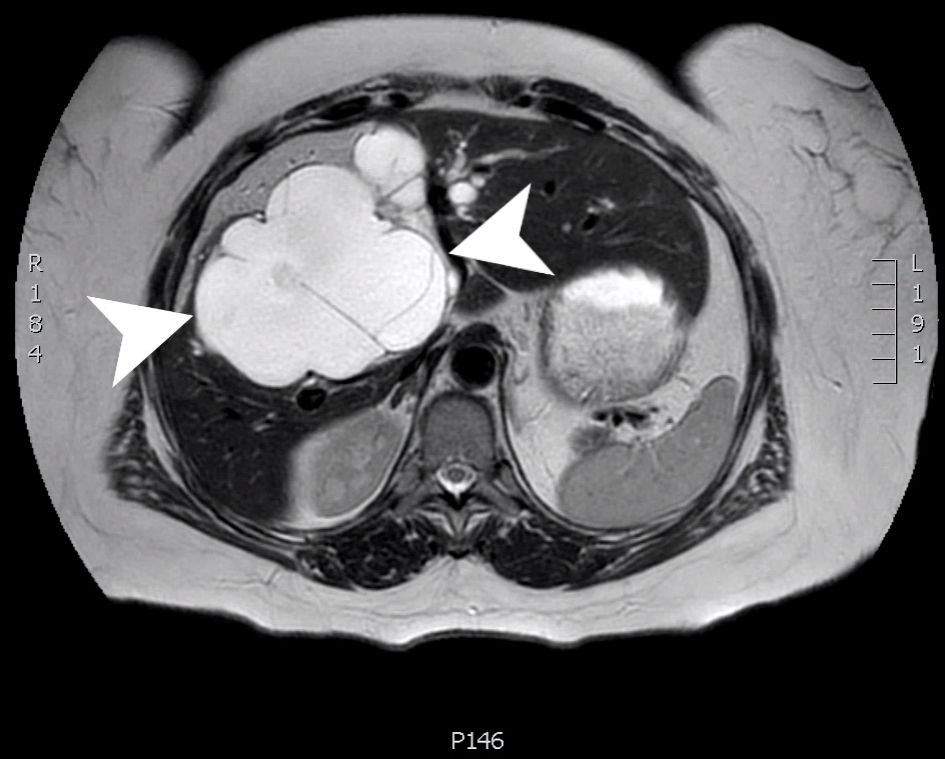 Click for large image | Figure 4. An axial slice from a T2 weighted MRI scan with Eovist contrast. A large, multiloculated cystic structure (arrow heads) within the central liver (segments 4, 5 and 8) measuring 12.5 × 8.9 × 2.1 cm. MRI: magnetic resonance imaging. |
The patient was sent to gastroenterology for an ERCP where two 7-French stents were placed in the right and left bile ducts to facilitate surgical removal of the cyst. Extrinsic compression of the common bile duct was noted as well.
Due to the patient’s worsening symptoms and enlarging cyst on imaging, surgery was once again offered to the patient. Cystic resection was difficult due to the previous unroofing and enucleation of liver cyst where adhesions had developed. Complete resection of the cyst with formal central hepatectomy was performed. On the final pathology report, many sections showed cystic structures lined by cuboidal to columnar epithelial cells with bland, basally oriented nuclei, and an ovarian-type stroma with elongated and undulating nuclei underlying the epithelium. This was consistent with a multiloculated BCA, grossly measuring 10 cm in greatest dimension, with surgical margins negative for cystadenoma.
Follow-up and outcomes
The patient did well postoperatively. Her most recent CT showed no recurrence of BCA, with evidence of central hepatectomy and compensatory hypertrophy (Fig. 5).
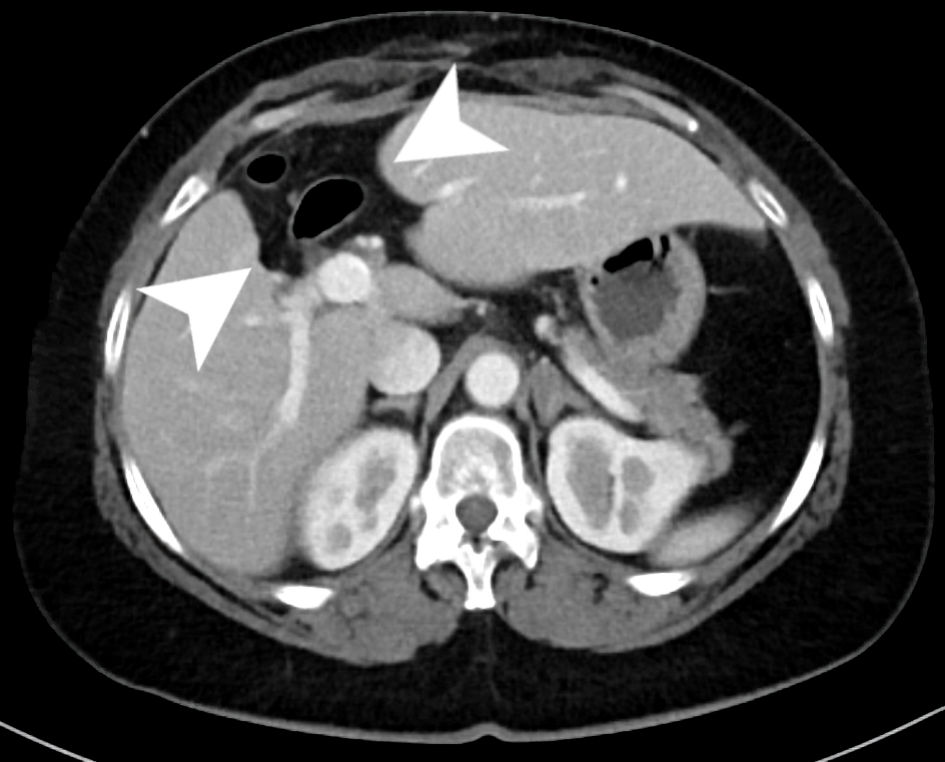 Click for large image | Figure 5. An axial slice from the venous phase of a CT scan with contrast. The scan shows chronic postoperative changes of a prior central hepatectomy within the inferior aspects of segments 4B and 8 (arrow heads) with evidence of compensatory hypertrophy of both the right and left hemi-liver. CT: computed tomography. |
| Discussion | ▴Top |
BCAs are rare, benign, tumors associated with the liver, and infrequently, the extrahepatic bile ducts. BCA and BCAC are tumors arising from the biliary epithelium. BCAs tend to occur in middle-aged women [2, 9-12] and have the potential for malignant transformation. This risk for transformation is thought to be as high as 20% to 30% [13]. Moreover, there have been a few case reports of benign cystadenomas transforming into cystadenocarcinomas [3, 14]. These instances further highlight their potential for malignant growth. While BCAs tend to occur more often in women, other studies have noted that BCACs tend to occur more in men [9, 10].
There is some discrepancy in the literature in terms of the prevalence of BCA. Most papers report an incidence of less than 5% of all hepatic cystic lesions [1, 2], but recent research estimates a lower incidence around 1.7% of intrahepatic cystic liver lesions [6]. Either way, these are rare tumors that should be considered when evaluating cystic lesions within the liver.
Most patients are asymptomatic at time of presentation and generally complain of vague abdominal symptoms [4-7]. One study reported that 88% of their patients with cystadenomas complained of upper abdominal pain or discomfort [11], while another stated that 74% of patients had abdominal pain [7]. Our patient presented with the usual complaints of abdominal pain and fullness. Interestingly, she also presented with the less often seen complaints of intermittent hyperbilirubinemia, dyspepsia, nausea, and vomiting. One study indicated that these rarer symptoms are likely due to pressure on adjacent viscera and organs [12] which would explain our patient’s symptoms as she presented with a fairly large mass.
The differential diagnosis for our patient included a complex cyst, BCA/cystadenocarcinoma, complex biloma, or a possible lymphangioma. Other hepatic cyst differentials can be quite exhaustive and include degeneration of metastatic tumors, hematomas, parasitic disease, hamartomas, Caroli’s disease, and polycystic liver disease [7, 15] to name a few.
Current diagnostic modalities highlight the difficulties in distinguishing biliary cystic tumors (BCT) from other hepatic lesions. Presently, there are no definitive, reliable clinical or imaging criterion for differentiating BCA from BCAC [15]. A clear example of this occurred in a study, where 16 patients were diagnosed preoperatively with BCA based on clinical and radiologic features, yet only six of these patients had BCA as their official diagnosis postoperatively [13]. Moreover, the nonspecific imaging features of BCA make accurate preoperative diagnosis difficult [16]. Despite these limitations, there are some general imaging findings that can be extrapolated and used with caution when a BCA is suspected. These include multilocular fluid collections with septations and debris on US [9], which was consistent with our patient’s presentation. Further, imaging studies with CT and MRI of our patient again aligned with the literature showing multilocular cystic processes [17]. The only definitive method for differentiating BCA from BCAC is pathologic examination. Overall, further research is needed to improve preoperative diagnostic specificity, which would facilitate timely interventions and appropriate treatment.
Laboratory values, such as cystic fluid analysis (CFA), have been proposed to aid in diagnosis of BCA and to help differentiate from other hepatic lesions. One study found that CFA can differentiate between simple cysts and BCA, since BCA exhibited elevated levels of carbohydrate antigen 19-9 (CA 19-9) while simple cysts had normal levels [18]. However, a different study found that cystic fluid CA 19-9 levels showed no significant difference between BCA and hepatic simple cysts [19]. Things get more difficult when differentiating between BCA and BCAC. One systematic review noted that liver function enzymes like aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) cannot be used to differentiate BCA from BCAC [20]. Furthermore, the same review found that elevated serum markers, like CA 19-9 and carcinoembryonic antigen (CEA), are suggestive of BCAC, but tumor markers within the normal range cannot rule out BCAC [20]. In general, further research is warranted in CFA, and blood markers, which might aid in differentiating hepatic lesions in the future.
There is more agreement in terms of histology, where BCT seem to fall into one of two categories: those that contain mesenchymal (ovarian-like) stroma and those that do not [2]. In a study observing 22 patients with intrahepatic BCAs, the cyst lining consisted of biliary epithelium +/- mesenchymal stroma [18]. Moreover, another study noted that all the cystadenomas were multilocular with benign cuboidal to columnar epithelium, and 44 (85%) had densely cellular spindle cell (“ovarian-like”) stromata [2]. Similar findings were found in our patient who showed cystic structures lined by cuboidal to columnar epithelial cells with bland, basally oriented nuclei, and an ovarian-type stroma with elongated and undulating nuclei underlying the epithelium.
Complete surgical resection is the preferred treatment method for BCA to prevent recurrence and limit the potential for malignant transformation [5-7, 11, 13, 18, 21, 22]. One study found that recurrence following surgical resection of BCT was relatively high, with nearly one in five patients experiencing recurrence. They also noted that recurrence was considerably higher among patients who underwent unroofing/fenestrations compared to formal hepatic resection [9]. In comparison, a systematic review reported a recurrence of 5.4% after resection for BCA and 4.8% after resection for BCAC, while recurrence after fenestration or marsupialization varied from 81.6% to 100% for both BCA and BCAC [20]. Another study noted 39 patients with either BCA (n = 35) or BCAC (n = 4) who underwent resection and reported a recurrence rate of 2.6% [6]. Further, one study investigating 10 cases of BCA and BCAC found that there was no recurrence in all patients after a median follow-up of 6 years [23].
Overall survival and prognosis for BCA seem to be favorable. One study examined a large, multi-institutional cohort of patients with BCT, and noted an overall survival of 84.2% [9]. Moreover, another paper concluded that prognosis of BCA and BCAC is excellent on most cases if a patient undergoes complete surgical resection [6]. Therefore, despite the potential for malignant transformation, complete resection yields favorable outcomes and prognosis for the patient.
Of note, current literature should be taken with a grain of salt, as most are single-center studies or based on a small sample size (often no more than 20 to 30 patients.) Therefore, future research on BCT is warranted.
Conclusions
In conclusion, BCAs often present clinically with nonspecific and variable symptoms. They have the potential for malignant transformation and thus require further workup. The literature varies on the prevalence of recurrence in BCA and more research is needed. Current imaging modalities have yet to clearly identify ways to differentiate BCA from other cystic hepatic lesions. Complete surgical resection is the preferred method for the prevention or recurrence and the transformation of malignancy, which also confers a favorable outcome.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Subject consent has been waived under the Institutional Review Board (IRB)-approved study protocol and the identity of the subject has been protected.
Author Contributions
NB and MD: study design, data collection, literature review, manuscript writing, figures preparation, and review of final manuscript. HO, EC, PM, AB, WK and DRJ: Study design, literature review, manuscript writing, and review of final manuscript.
Data Availability
Data supporting the findings of this study are available within the article.
| References | ▴Top |
- Walt AJ. Cysts and benign tumors of the liver. Surg Clin North Am. 1977;57(2):449-464.
doi pubmed - Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18(11):1078-1091.
doi pubmed - Woods GL. Biliary cystadenocarcinoma: Case report of hepatic malignancy originating in benign cystadenoma. Cancer. 1981;47(12):2936-2940.
doi pubmed - Soares KC, Arnaoutakis DJ, Kamel I, Anders R, Adams RB, Bauer TW, Pawlik TM. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg. 2014;218(1):119-128.
doi pubmed - Emre A, Serin KR, Ozden I, Tekant Y, Bilge O, Alper A, Gulluoglu M, et al. Intrahepatic biliary cystic neoplasms: Surgical results of 9 patients and literature review. World J Gastroenterol. 2011;17(3):361-365.
doi pubmed - Chen YW, Li CH, Liu Z, Dong JH, Zhang WZ, Jiang K. Surgical management of biliary cystadenoma and cystadenocarcinoma of the liver. Genet Mol Res. 2014;13(3):6383-6390.
doi pubmed - Thomas KT, Welch D, Trueblood A, Sulur P, Wise P, Gorden DL, Chari RS, et al. Effective treatment of biliary cystadenoma. Ann Surg. 2005;241(5):769-773; discussion 773-765.
doi pubmed - Stanley J, Vujic I, Schabel SI, Gobien RP, Reines HD. Evaluation of biliary cystadenoma and cystadenocarcinoma. Gastrointest Radiol. 1983;8(3):245-248.
doi pubmed - Arnaoutakis DJ, Kim Y, Pulitano C, Zaydfudim V, Squires MH, Kooby D, Groeschl R, et al. Management of biliary cystic tumors: a multi-institutional analysis of a rare liver tumor. Ann Surg. 2015;261(2):361-367.
doi pubmed - Wang C, Miao R, Liu H, Du X, Liu L, Lu X, Zhao H. Intrahepatic biliary cystadenoma and cystadenocarcinoma: an experience of 30 cases. Dig Liver Dis. 2012;44(5):426-431.
doi pubmed - Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200(5):727-733.
doi pubmed - Ishak KG, Willis GW, Cummins SD, Bullock AA. Biliary cystadenoma and cystadenocarcinoma: report of 14 cases and review of the literature. Cancer. 1977;39(1):322-338.
doi pubmed - Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30(8):1560-1566.
doi pubmed - Matsuoka Y, Hayashi K, Yano M. Case report: malignant transformation of biliary cystadenoma with mesenchymal stroma: documentation by CT. Clin Radiol. 1997;52(4):318-321.
doi pubmed - Del Poggio P, Buonocore M. Cystic tumors of the liver: a practical approach. World J Gastroenterol. 2008;14(23):3616-3620.
doi pubmed - Soochan D, Keough V, Wanless I, Molinari M. Intra and extra-hepatic cystadenoma of the biliary duct. Review of literature and radiological and pathological characteristics of a very rare case. BMJ Case Rep. 2012;2012:bcr0120125497.
doi pubmed - Averbukh LD, Wu DC, Cho WC, Wu GY. Biliary mucinous cystadenoma: a review of the literature. J Clin Transl Hepatol. 2019;7(2):149-153.
doi pubmed - Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136(4):926-936.
doi pubmed - Choi HK, Lee JK, Lee KH, Lee KT, Rhee JC, Kim KH, Jang KT, et al. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44(4):289-293.
doi pubmed - Klompenhouwer AJ, Ten Cate DWG, Willemssen F, Bramer WM, Doukas M, de Man RA, Ijzermans JNM. The impact of imaging on the surgical management of biliary cystadenomas and cystadenocarcinomas; a systematic review. HPB (Oxford). 2019;21(10):1257-1267.
doi pubmed - Pitchaimuthu M, Aidoo-Micah G, Coldham C, Sutcliffe R, Roberts JK, Muiesan P, Isaac J, et al. Outcome following resection of biliary cystadenoma: a single centre experience and literature review. Int J Hepatol. 2015;2015:382315.
doi pubmed - Kubota E, Katsumi K, Iida M, Kishimoto A, Ban Y, Nakata K, Takahashi N, et al. Biliary cystadenocarcinoma followed up as benign cystadenoma for 10 years. J Gastroenterol. 2003;38(3):278-282.
doi pubmed - Fragulidis GP, Vezakis AI, Konstantinidis CG, Chondrogiannis KK, Primetis ES, Kondi-Pafiti A, Polydorou AA. Diagnostic and therapeutic challenges of intrahepatic biliary cystadenoma and cystadenocarcinoma: a report of 10 cases and review of the literature. Int Surg. 2015;100(7-8):1212-1219.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
