| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Original Article
Volume 12, Number 1, March 2022, pages 7-14
Effect of Upper Limb Warming Blanket on Body Temperature Control During Total Hip Arthroplasty and Total Knee Arthroplasty
Yuta Mitobea, h, Kenji Nakagawab, Yasuko Babac, Tomomi Yoshiokad, Yuri Yamaguchie, Takeshi Itouf, Kiyoyasu Kurahashig
aGraduate School of Health and Welfare Science, International University of Health and Welfare, Tokyo, Japan
bDepartment of Nursing, International University of Health and Welfare, Mita Hospital, Tokyo, Japan
cDepartment of Anesthesiology, International University of Health and Welfare, Mita Hospital, Tokyo, Japan
dDepartment of Nursing, Faculty of Health Science, Tokoha University, Shizuoka, Japan
eDepartment of Anesthesiology, Yokohama Municipal Citizen’s Hospital, Kanagawa, Japan
fDepartment of Nursing, Tokyo Metropolitan Bokutoh Hospital, Tokyo, Japan
gDepartment of Anesthesiology, Intensive Care Medicine International University of Health and Welfare, Chiba, Japan
hCorresponding Author: Yuta Mitobe, Graduate School of Health and Welfare Science, International University of Health and Welfare, Tokyo, Japan
Manuscript submitted February 16, 2022, accepted March 2, 2022, published online March 29, 2022
Short title: Body Temperature Control During THA and TKA
doi: https://doi.org/10.14740/jcs456
| Abstract | ▴Top |
Background: At our research facility, the body temperature is constantly monitored using a continuous-measuring ear thermometer, i.e., Nipro CE Thermo (hereafter CE Thermo). However, this temperature control assumes that the CE Thermo itself does not get affected, thus warranting the identification of influencing factors for temperature control based on their influence on the CE Thermo. We aimed to evaluate the tympanic membrane temperature of patients undergoing lower limb surgery under general anesthesia, and to explore body temperature fluctuations during anesthesia based on the tympanic membrane temperature.
Methods: We selected patients who underwent total hip arthroplasty (THA) and total knee arthroplasty (TKA) for lower extremities at the Department of Orthopedic Surgery, Okinawa Tokushukai Kamagaya General Hospital between January 2017 and March 2020. We conducted a retrospective observational study of 173 patients undergoing THA and TKA for lower extremities at a single institution.
Results: We calculated and compared the tympanic temperature measurements at 5, 10, 15, 20, 25, 30, 35, and 40 min between the groups to predict the factors of temperature variation. The results of tympanic temperature measurements were analyzed using two-way analysis of variance. At the beginning of the measurement, the THA group demonstrated significantly lower values than the TKA group (P = 0.001).
Conclusions: The above results necessitate further clarification of the effect of temperature fluctuation, including surgical factors, on prognosis.
Keywords: Eardrum temperature; CE Thermo; Upper limb warming air blanket; THA; TKA
| Introduction | ▴Top |
Effect of hypothermia during anesthesia
The hypothalamus controls the body temperature of humans, a type of thermostatic mammal, even during cyclic fluctuations through thermoregulatory responses, such as sweating, vasoconstriction, and thermogenesis [1]. Anesthesia alters the physiological regulation of body temperature, and the body becomes susceptible to the influence of ambient temperature (metazoan state). The change in intraoperative body temperature is attributed to anesthesia-induced suppression of sympathetic nerves and the dilation of peripheral blood vessels, which result in the transfer of central heat to the periphery and a decrease in the core temperature [2]. An exposure to a cold environment beyond the limits of autonomous thermoregulation or a decline in the body’s ability to maintain its temperature decreases the temperature to a level below the lower limit of the homeostatic body temperature, thereby causing various problems in body functions and multiple organ failure. Hypothermia during anesthesia, postoperative infection [3], increased intraoperative blood loss [4, 5], perioperative myocardial infarction [6], postoperative tremor [7], and coagulopathy [8] can cause numerous complications. Therefore, methods to prevent hypothermia during anesthesia include the use of a hot air heating system [8, 9], covering of the face and head [10], forced heating by intravenous infusion [9-11], and the administration of amino acid preparations [5, 6, 12].
Intraoperative temperature control of total hip arthroplasty (THA) and total knee arthroplasty (TKA)
Temperature control during anesthesia dates back to 1900 and 1960, when fever and convulsions during ether anesthesia were reported in England and the first familial case of malignant hyperthermia was reported in Australia, respectively. In this study, we selected the rectal, bladder, and tympanic membrane temperatures for temperature control in the operating room depending on the case. The continuous-measuring ear thermometer Nipro CE Thermo® (hereinafter referred to as CE Thermo), which can measure the tympanic membrane temperature, was used most frequently. The tympanic membrane temperature shares blood with the hypothalamus, which controls body temperature; therefore, the internal carotid artery blood temperature can be used to measure body temperature close to the central temperature [13]. Bladder temperature is dependent on urine [14], whereas tympanic temperature is one of the nuclear body temperatures, the highest temperature inside the body regulated within a certain temperature range, and is correlated with esophageal and rectal temperatures [15]. A previous report comparing the THA and TKA groups for temperature changes [16] demonstrated that the esophageal temperature was significantly lower in the THA group than that in the TKA group. The results suggested that unilateral ejection may have prevented a decrease in body temperature in the TKA group during ejection. However, esophageal and tympanic membrane temperatures were significantly lower in the TKA group following the release of ejection, whereas the bladder and rectal temperatures were not lowered.
Purpose of the study
Our purpose was to determine body temperature fluctuations during anesthesia based on tympanic membrane temperature in orthopedic patients undergoing lower extremity surgery.
| Materials and Methods | ▴Top |
Research design
This is a retrospective observational study using electronic medical records.
Research subjects
We selected patients who underwent THA and TKA for lower extremities at the Department of Orthopedic Surgery, Okinawa Tokushukai Kamagaya General Hospital between January 2017 and March 2020. The inclusion criteria were as follows: 1) Patients who underwent orthopedic THA and TKA at the Okinawa Tokushukai Kamagaya General Hospital between January 2017 and March 31, 2020; 2) Patients whose tympanic membrane temperature was measured during the surgery; and 3) Patients undergoing general anesthesia and combined epidural anesthesia. The exclusion criteria were as follows: 1) aged < 20 years; 2) undergoing spinal anesthesia; 3) with missing tympanic temperature data; 4) with missing laboratory data; and 5) died during hospitalization.
Basic patient data
We examined the following basic data of all patients: tympanic membrane temperature during anesthesia, the duration and method of anesthesia, the American Society of Anesthesiologists-Physical Status (ASA-PS), age, sex, body mass index (BMI), height, weight, surgical procedure, the name of disease, the length of hospitalization, and the presence of shivering (Table 1).
 Click to view | Table 1. Patient Background and Method of Anesthesia |
Blood data
We assessed the results of blood tests performed by the anesthesiologist at the preoperative anesthesiology outpatient clinic to evaluate the general condition of all patients. Blood data items included the following: albumin (Alb), blood urea nitrogen, creatinine (Cre), C-reactive protein (CRP), platelet count, hematocrit (HT), white blood cell (WBC), red blood cell (RBC), prothrombin time (PT), activated partial thromboplastin time (APTT), prothrombin time-international normalized ratio (PTINR), creatine kinase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, γ-glutamyl transpeptidase (γ-GTP), total serum protein (TP), total bilirubin, sodium (Na), potassium (K), chloride (Cl), and glycosylated hemoglobin.
| Statistical analysis | ▴Top |
We performed the Mann-Whitney U test and Fisher’s exact test to compare the THA and TKA groups under general anesthesia. In addition, a univariate analysis was performed to examine variations in tympanic membrane temperature in each group over time. We used version 2.6-1 of the EZR Commander for all statistical analyses, and a P value < 0.05 was considered statistically significant.
Ethical considerations
Ethical considerations for the research participants were in accordance with the tenets of the Declaration of Helsinki and the ethical guidelines for medical research involving human subjects, by notifying and disclosing information in advance and ensuring that all participants had the opportunity to refuse participation (opt-out). This study was conducted with the approval of the Tokushukai Group Joint Ethical Review Committee (approval number: TGE01496-064, June 25, 2020).
| Results | ▴Top |
Overall results
During the study, 173 patients were diagnosed with osteoarthritis and rheumatoid arthritis of the hip and knee joints and were eligible for THA and TKA. Seventeen patients met the exclusion criteria, namely three with spinal anesthesia, 11 with missing general anesthesia tympanic temperature data, and three with missing laboratory data. Eventually, 156 patients were included in the analysis; 105 and 51 patients were divided into the THA group and TKA group, respectively (Fig. 1). We performed all analyses using anesthesia and the ASA-PS classification (Table 1).
 Click for large image | Figure 1. Flowchart for patient selection. During the study, 173 patients were diagnosed with osteoarthritis and rheumatoid arthritis of the hip and knee joints and were eligible for THA and TKA. Eventually, 156 patients were included in the analysis; 105 and 51 patients were divided into the THA group and TKA group, respectively. THA: total hip arthroplasty; TKA: total knee arthroplasty. |
Patient background
The mean age of the THA and TKA group was 64.8 years (± 10.7) and 74.6 years (± 6.1), respectively (P = 0.01). The BMI of the THA group was significantly lower than that of the TKA group (P = 0.01). There were no significant differences in other items between the groups.
Comparison of preoperative blood test data
In the preoperative blood test data, there were no significant differences in the Alb, ALT, AST, Cl, Cre, CRP, γ-GTP, Hb, HT, K, Na, PT, APTT, PTINR, PT, RBC, TP, and WBC between the two groups (Table 2).
 Click to view | Table 2. Preoperative Blood Test Data |
Comparison of intraoperative body temperature
We calculated and compared the tympanic temperature measurements at 5, 10, 15, 20, 25, 30, 35, and 40 min between the groups to predict the factors of temperature variation (Table 3). The results of tympanic temperature measurements were analyzed using two-way analysis of variance (Fig. 2). At the beginning of the measurement, the THA group demonstrated significantly lower values than the TKA group (P = 0.001).
 Click to view | Table 3. Comparison From the Start of Tympanic Membrane Temperature Measurement to the End of Tympanic Membrane Temperature Measurement |
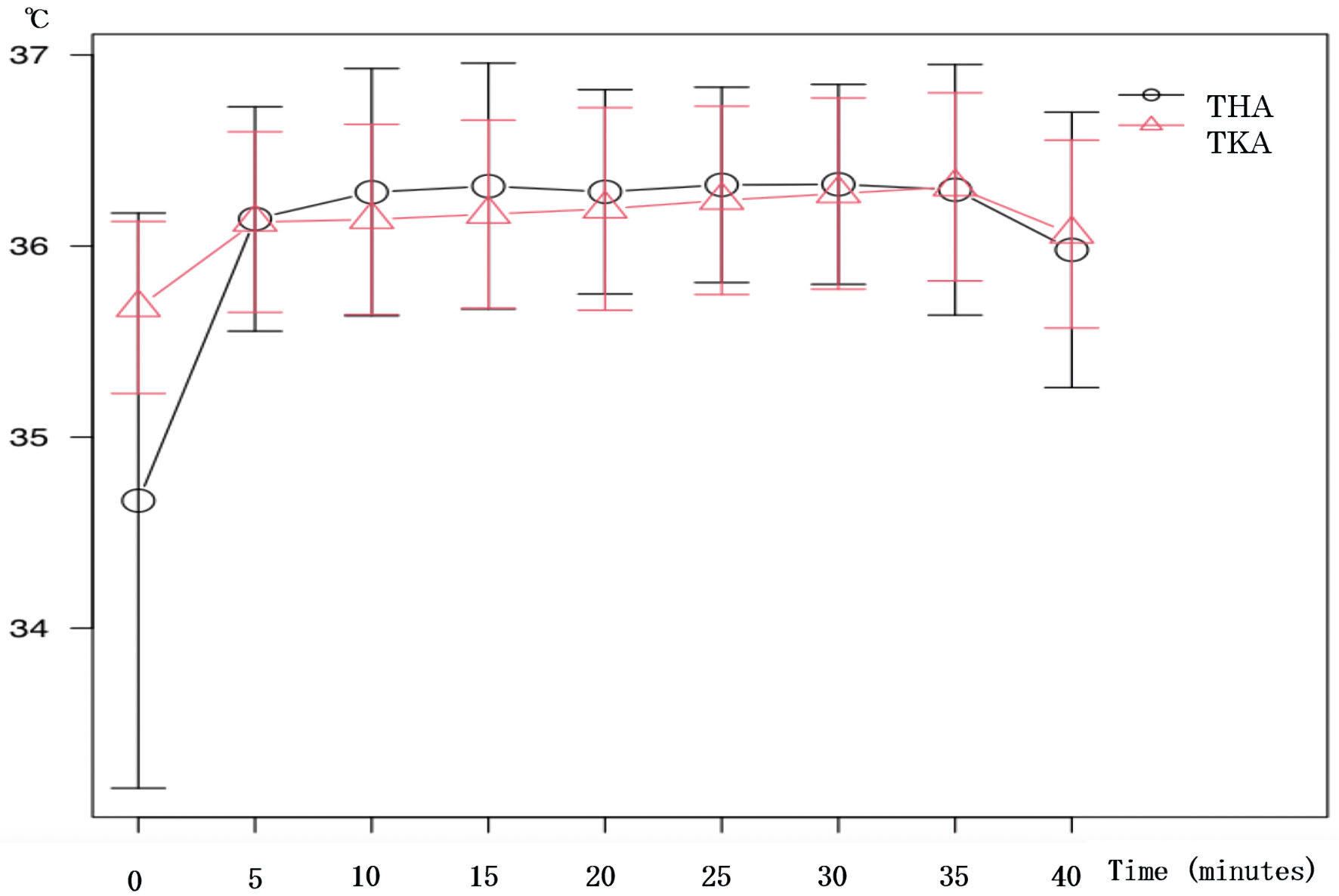 Click for large image | Figure 2. Trends in tympanic membrane temperature for THA and TKA groups. The results of tympanic temperature measurements were analyzed using two-way analysis of variance. At the beginning of the measurement, the THA group demonstrated significantly lower values than the TKA group (P = 0.001). THA: total hip arthroplasty; TKA: total knee arthroplasty. |
Changes in tympanic membrane temperature
Table 3 summarizes changes in tympanic membrane temperature in the THA and TKA groups. Both groups demonstrated low values at the beginning of tympanic membrane temperature measurement owing to the product of CE Thermo; however, the THA group revealed lower body temperature at the beginning.
Changes in tympanic membrane temperature in THA and TKA groups
Figure 2 depicts the trends of tympanic membrane temperature in the THA group (≤ 36 °C) and TKA group (≥ 36 °C). Up to 5 min following the measurement, 113 patients in the THA group and 43 patients in the TKA group exhibited a tympanic membrane temperature ≥ 36 °C and ≤ 36 °C, respectively. From 10 min to the end of the tympanic membrane temperature measurement, the number of patients in the TKA group with a tympanic membrane temperature of 36 °C or higher was higher than that in the TKA group.
Incidence of shivering
Only one patient in the THA group had shivering in both the THA and TKA groups in the electronic medical record. The mean intraoperative tympanic temperature that induced shivering was 36.7 °C.
| Discussion | ▴Top |
Perioperative temperature management includes rectal, bladder, pharyngeal, and tympanic membrane temperatures. The tympanic membrane temperature has been used as a temperature monitor during all surgeries since January 2017 at the research institution. This is because the thermometer is inserted into the external ear, is less invasive, and monitors changes in the core temperature to obtain a realistic temperature. Preventing perioperative hypothermia has various benefits, including shortening the duration of surgery, improving the complication rate, and shortening the length of hospitalization [17-19]. The use of the warm air heating system can reduce the risks that occur in the perioperative period. Predicting the relationship between variations in the tympanic membrane temperature and the warm air heating system in different surgical positions and techniques can facilitate early detection and respond to perioperative complications.
Frequency of perioperative hypothermia
In the 90s, Sessler et al reported on perioperative hypothermia in healthy subjects and surgical patients [20-22]. In 1996, a prospective, randomized controlled study of the relationship between perioperative hypothermia and blood loss in 60 patients undergoing hip arthroplasty, with and without general anesthesia, reported that active warming reduced blood loss and blood transfusion [4]. In this study, the TKA and THA groups received active heating, and the average blood loss was 50 mL, similar to previous results. The most likely cause was a decrease in platelet function and coagulability owing to reduced body temperature. The relationship between perioperative hypothermia, blood loss, and blood transfusion was such that a difference of only 1 °C of the central temperature increased blood loss and the risk of blood transfusion by approximately 16% and 22%, respectively, in orthopedic surgery, gynecology, cardiovascular surgery, and laparotomy [23]. A prospective randomized controlled study of 200 patients scheduled for lower abdominal gastrointestinal surgery examined perioperative hypothermia, the incidence of infection, wound healing, and hospital stay, and reported on a significant improvement following active intraoperative warming [7]. In 2001, 30 min of preoperative whole-body warming suppressed surgical site infection and reduced the use of postoperative antibiotics even in small surgeries lasting for approximately 1 h, which ensured a clean surgical field [24].
The average time for THA and TKA was 64.9 min and 67.1 min, respectively. Cases of perioperative hypothermia induced by both techniques were absent because of the short surgical duration, as in the previous study. Perioperative hypothermia is usually defined as a core body temperature < 36 °C [25], and hypothermia without prophylactic warming devices reportedly occurs in 50-90% of the surgical patients [26]. In this study, the THA and TKA groups were compared on the basis of 36 °C from 5 min to 40 min following the beginning of tympanic membrane temperature measurement using a heating device (Table 3). Following 5 min of the measurement, 113 patients and 43 patients in the THA and TKA group exhibited a tympanic membrane temperature ≤ 36 °C and ≥ 36 °C, respectively. Higher number of patients in the THA group with body temperatures < 36 °C may be attributed to body movements associated with intraoperative manipulation. The intraoperative positioning of the patients for TKA took < 10 min from the beginning of anesthesia to that of the surgery. Moreover, the supine position minimized the exposure of the trunk, which supposedly resulted in less fluctuation of the tympanic membrane temperature. By contrast, THA was performed in the lateral supine position, and the time from the beginning of anesthesia to that of the surgery was < 20 min. In addition, the trunk was exposed considering the importance of thoroughly assessing the nerve compression site and the range of motion of the joints during patient immobilization. The aforementioned factors may have resulted in body temperatures < 36 °C.
Hypothermia owing to the effects of general anesthesia
During anesthesia, the central temperature rapidly decreases from 0.5 °C to 1.5 °C within 1 h of induction, except for malignant hyperthermia, fever, and depressive fever [27]. Following anesthetic administration, peripheral blood vessels that regulate the body temperature rapidly dilate owing to a significant decrease in the vasoconstriction threshold; however, they remain constricted before anesthesia induction, and heat gets stored in the central nervous system. Thus, the stored heat shifts to the periphery, thereby causing a rapid decrease in the central temperature [28]. In this study, the average time from the beginning of anesthesia to its end was 111 min; thus, despite the body temperature being lower during anesthesia than that before its induction, it could be predicted that it was due to the effect of anesthesia vasodilation. Following a temporary decrease in body temperature after anesthesia induction, it tends to increase during anesthesia, which supposedly affects intraoperative fluctuations. Causes of intraoperative hypothermia include [28] redistributive hypothermia, radiation, transpiration, conduction, and convection. The initial sudden drop in the body temperature followings general anesthesia is termed redistributive hypothermia, in which the core temperature during the entry into the operating room is approximately 37 °C; however, the peripheral temperature is as low as 31 - 35 °C. Heat gets dispersed by the blood; under anesthesia, the blood vessels dilate and the heat gets distributed to the periphery, which decreases the core temperature. Furthermore, heat loss occurs approximately 1 h after the induction of anesthesia, during which the body temperature decreases because of differences between redistribution and heat loss. Following anesthesia, heat loss occurs by radiation, evaporation, conduction, and convection. Calorific production occurs simultaneously; however, metabolic heat production rapidly decreases under anesthesia. Therefore, the calorific output becomes negative approximately 1 h following anesthesia induction, concomitant with heat loss from the skin. Vasoconstriction occurs following a decrease in body temperature, and the core temperature plateaus. Usually, a decrease in core temperature causes vasoconstriction, and the body temperature is precisely controlled at 36 - 37 °C. In this study, a large percentage of patients in the THA group exhibited an intraoperative body temperature < 36 °C, whereas a large percentage of those in the TKA group exhibited a temperature ≥ 36 °C, despite similar conditions.
Limitations and challenges
This study only included data on current diseases, thus warranting the collection of data on underlying diseases associated with body temperature. Women and THA increase the risk of hypothermia [25]. In the present study, there were more women than men, which may have affected the results of the tympanic temperature variation. However, further investigation is required to determine a difference in body temperature and sex ratio. In this study, we initially collected retrospective information from the electronic medical records, followed by a comparative study according to the type of surgery; however, we did not include the name of the disease. Thus, we may not have been able to completely evaluate the results. Third, we conducted a single-center study with fewer patients. Fourth, it was necessary to collect information on lifestyle habits owing to its possible association with the current disease, and this relationship needs to be clarified. Fifth, we collected preoperative but not postoperative blood data; therefore, we were unable to compare preoperative and postoperative blood test data. In the future, we should clarify the extent by which intraoperative temperature fluctuations affect postoperative blood data. Sixth, the intraoperative body temperature was solely based on tympanic temperature measurement results. This necessitates examining the results of intraoperative body temperature, which is more accurate than other methods of measuring central temperature, such as esophageal, rectal, and bladder temperatures. Moreover, the manufacturer and results of our study suggested that the body temperature was not reliable for approximately 10 min immediately before the beginning of tympanic temperature measurement, thus warranting the measurement of body temperature before the beginning of surgery in the future. Hypothermia is associated with an increase in estimated blood loss [25]. However, there was no significant difference between the groups because all patients underwent postoperative blood transfusion. Therefore, researchers may determine intraoperative hypothermia and its effect on postoperative complications by examining the relationship between intraoperative blood loss and blood transfusion.
Conclusions
We measured tympanic membrane temperature using a CE thermometer in patients who underwent THA and TKA, besides identifying the factors that affected tympanic membrane temperature fluctuation. The THA surgery demonstrated lower values than the TKA surgery, and we were able to infer the cause of the variation in the tympanic membrane temperature. Considering the short surgery time for THA and TKA at our institution, the effect of temperature variation was insignificant; however, the general average operative time may be useful in predicting perioperative hypothermia. This necessitates determining the effect of body temperature fluctuations, including surgical factors, on disease prognosis.
Acknowledgments
We thank Okinawa Tokushukai Kamagaya General Hospital for the helpful advice on the analysis methods.
Financial Disclosure
This work was supported by JSPS KAKENHI Grant (number: JP1919577).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consents were obtained from all patients.
Author Contributions
YM, KN has designed and performed the study. TY, YY and TI have drafted the manuscript and did critical editing. YM, and KN have assisted and supported in sample collection and subsequent analysis with statistics. KK and YB have carefully supervised this manuscript preparation and writing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
Alb: albumin; ALT: alanine aminotransferase; APTT: activated partial thromboplastin time; AST: aspartate aminotransferase; BMI: body mass index; CE Thermo: Nipro CE Thermo; Cl: chloride; Cre: creatinine; CRP: C-reactive protein; HT: hematocrit; K: potassium; Na: sodium; PT: prothrombin time; PTINR: prothrombin time-international normalized ratio; RBC: red blood cell; THA: total hip arthroplasty; TKA: total knee arthroplasty; TP: total serum protein; WBC: white blood cell
| References | ▴Top |
- Lenhardt R, Kurz A, Sessler DI. Thermoregulation and hyperthermia. Acta Anaesthesiol Scand Suppl. 1996;109:34-38.
- Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387(10038):2655-2664.
doi - Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Tem-perature Group. N Engl J Med. 1996;334(19):1209-1215.
doi pubmed - Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347(8997):289-292.
doi - Widman J, Hammarqvist F, Sellden E. Amino acid infusion induces thermogenesis and reduces blood loss during hip arthroplasty under spinal anesthesia. Anesth Analg. 2002;95(6):1757-1762.
doi pubmed - Frank SM, Beattie C, Christopherson R, Norris EJ, Perler BA, Williams GM, Gottlieb SO. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;78(3):468-476.
doi pubmed - Eberhart LHJ, Doderlein F, Eisenhardt G, Kranke P, Sessler DI, Torossian A, Wulf H, et al. Independent risk factors for postoperative shivering. Anesth Analg. 2005;101(6):1849-1857.
doi pubmed - Cavallini M, Baruffaldi Preis FW, Casati A. Effects of mild hypothermia on blood coag-ulation in patients undergoing elective plastic surgery. Plast Reconstr Surg. 2005;116(1):316-321; discussion 322-313.
doi pubmed - Kamiya K, Asahi T, Higuchi A, et al. Changes in body temperature due to the use of lower limb turniquettes: Effects of room temperature and thermal insulation measures. Anes-thesia. 2005;54:138-143.
- Kamitani K, Higuchi A, Takebayashi T, Miyamoto Y, Yoshida H. Covering the head and face maintains intraoperative core temperature. Can J Anaesth. 1999;46(7):649-652.
doi pubmed - Yamauchi M, Nakayama Y, Yamakage M, Tsuchida H, Iwasaki H, Namiki A. [Preven-tive effect of fluid warmer system on hypothermia induced by rapid intravenous infusion]. Masui. 1998;47(5):606-610.
- Kasai T, Nakajima Y, Matsukawa T, Ueno H, Sunaguchi M, Mizobe T. Effect of pre-operative amino acid infusion on thermoregulatory response during spinal anaesthesia. Br J Anaesth. 2003;90(1):58-61.
doi pubmed - Yamagishi A, Toyama H, Tobise F, et al. Usefulness of non-contact continuous tympanic membrane temperature measurement device (Nipro CE Thermo) in surgery using artificial heart lung. Anesthesia. 2012;61:896-900.
- Horrow JC, Rosenberg H. Does urinary catheter temperature reflect core temperature during cardiac surgery? Anesthesiology. 1988;69(6):986-989.
doi pubmed - Cork RC, Vaughan RW, Humphrey LS. Precision and accuracy of intraoperative temper-ature monitoring. Anesth Analg. 1983;62(2):211-214.
doi pubmed - Mizokami M, Yanagimoto M, Harada J. Variation of body temperature with use of tour-niquet during lower limb surgery. Clinical Body Temperature. 1994;14:68-72.
- Scott EM, Leaper DJ, Clark M, Kelly PJ. Effects of warming therapy on pressure ul-cers—a randomized trial. AORN J. 2001;73(5):921-927, 929-933, 936-928.
doi - Casati A, Fanelli G, Ricci A, Musto P, Cedrati V, Altimari G, Baroncini S, et al. Short-ening the discharging time after total hip replacement under combined spinal/epidural anes-thesia by actively warming the patient during surgery. Minerva Anestesiol. 1999;65(7-8):507-514.
- Moola S, Lockwood C. The effectiveness of strategies for the management and/or pre-vention of hypothermia within the adult perioperative environment: systematic review. JBI Libr Syst Rev. 2010;8(19):752-792.
doi pubmed - Matsukawa T, Kurz A, Sessler DI, Bjorksten AR, Merrifield B, Cheng C. Propofol line-arly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82(5):1169-1180.
doi pubmed - Sessler DI, Schroeder M, Merrifield B, Matsukawa T, Cheng C. Optimal duration and temperature of prewarming. Anesthesiology. 1995;82(3):674-681.
doi pubmed - Ozaki M, Kurz A, Sessler DI, Lenhardt R, Schroeder M, Moayeri A, Noyes KM, et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology. 1994;81(2):282-288.
doi pubmed - Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypother-mia on blood loss and transfusion requirement. Anesthesiology. 2008;108(1):71-77.
doi pubmed - Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the inci-dence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876-880.
doi - Frisch NB, Pepper AM, Rooney E, Silverton C. Intraoperative hypothermia in total hip and knee arthroplasty. Orthopedics. 2017;40(1):56-63.
doi pubmed - Young VL, Watson ME. Prevention of perioperative hypothermia in plastic surgery. Aesthet Surg J. 2006;26(5):551-571.
doi pubmed - Ikeda T, Kazama F. Prevention of hypothermia during anesthesia. Journal of the Japanese Association of Surgical Surgeons. 2004;25:115-117.
- Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92(2):578-596.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
