| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 11, Number 4, December 2021, pages 87-96
An Adenoneuroendocrine Collision Tumor of the Pancreas: A Case Report
Christine Chunga , Muhammad Bassel Darwishb
, Patrick James McLarenb, Annie Laurie Benzieb, Wareef Kabbanic, Edward Chob, d, Houssam Osmanb, d, Dhiresh Rohan Jeyarajahb, d, e
aDepartment of Surgery, HCA HealthOne Swedish Medical Center, Englewood, CO, USA
bDepartment of Surgery, Methodist Richardson Medical Center, Richardson, TX, USA
cDepartment of Pathology, Methodist Health System, Dallas, TX, USA
dDepartment of Surgery, TCU/UNTHSC School of Medicine, Fort Worth, TX, USA
eCorresponding Author: Dhiresh Rohan Jeyarajah, Department of Surgery, Methodist Richardson Medical Center, 2805 East President George Bush Highway, Richardson, TX 75082, USA
Manuscript submitted June 1, 2021, accepted June 14, 2021, published online October 26, 2021
Short title: Pancreatic Collision Tumor
doi: https://doi.org/10.14740/jcs440
| Abstract | ▴Top |
Collision tumors, specifically adenoneuroendocrine tumors of the pancreas, are rare. Here we present a 57-year-old female patient with neurofibromatosis who presented with 2 months of nausea, vomiting, and epigastric pain. Imaging and endoscopy revealed severe narrowing of the third portion of the duodenum and an incidental adrenal mass. She underwent an exploratory laparotomy, right adrenalectomy, and pancreaticoduodenectomy. Pathology revealed a 4.7 cm pT3bN2M0R1 peri-ampullary combined poorly differentiated carcinoma and grade 1 neuroendocrine tumor. Due to the small number of reported cases of mixed adenoneuroendocrine carcinoma of the pancreas, clinical behavior remains unclear and definitive management approaches have not been established.
Keywords: Collision tumor; Pancreas; Pancreaticoduodenectomy; Pancreatic ductal adenocarcinoma; Neuroendocrine tumor
| Introduction | ▴Top |
A collision tumor is a neoplastic mass made up of two or more distinct cell populations that maintain distinct borders. Specifically, a mixed adenoneuroendocrine carcinoma (MANEC) is where a tumor has at least 30% adenocarcinoma component and at least 30% neuroendocrine carcinoma component [1].
Pancreatic adenocarcinoma (PDAC) is rare but one of the most common malignant pancreatic tumors, whereas neuroendocrine pancreatic tumors are extremely rare at 1-2% [2]. The incidence of the two as a combined neoplasm ranges only from 0.06% to 0.20% [2].
Herein we present a 57-year-old female patient with 2 months of nausea, vomiting, and epigastric abdominal pain. Imaging and endoscopic evaluation revealed extrinsic compression on the third portion of her duodenum. She underwent an operation in which a pancreatic mass was resected and pathology revealed a MANEC with both PDAC and neuroendocrine tumor (NET) components with multiple foci of gastrointestinal stromal tumor (GIST) found in the duodenum.
| Case Report | ▴Top |
This is a 57-year-old female patient with a past medical history of lung cancer after partial lobectomy 15 years prior to presentation, oxygen dependent chronic obstructive pulmonary disease (COPD), previous smoker (quit a year prior to presentation), and familial neurofibromatosis (unknown type) who presented with 2 months of nausea, vomiting, and epigastric abdominal pain. She was initially seen at an outside hospital where a computed tomography (CT) scan revealed a mass in the third portion of the duodenum, a distended stomach, and an incidental right adrenal mass. She underwent an upper endoscopy and endoscopic ultrasound which revealed duodenal stenosis with significant mucosal edema concerning for extrinsic compression or malignancy, and peripheral neoplastic lymph nodes. Fine-needle aspiration (FNA) biopsies were obtained and were concerning for malignancy. The patient was then referred to our hospital.
On presentation, she complained of abdominal pain with oral intake, which caused non-bloody emesis and in turn, relieved the pain. She also complained of constipation. On examination, her blood pressure was 179/84 mm Hg, heart rate was 68 bpm, respiratory rate was 18, oxygen saturation was 100%, and temperature was 98.7 °F. There were numerous neurofibromas noted mainly on the back and trunk and a few cafe au lait spots. The abdomen was soft but mildly tender to palpation. The rest of the examination was unremarkable. Labs revealed a CO2 of 36 mmol/L (range 22 - 31 mmol/L), an anion gap of 4 mEq/L (range 8 - 16 mEq/L), a blood urea nitrogen (BUN) of 4 mg/dL (range 10 - 25 mg/dL), a creatinine of 0.60 mg/dL (0.70 - 1.40 mg/dL), a phosphorus level of 2.3 (range 2.6 - 4.9 mg/dL), and an alanine aminotransferase (ALT) of 41 U/L (range < 35 U/L). CA19-9 and carcinoembryonic antigen (CEA) were both negative. All other lab values were within normal limits.
A CT of chest abdomen and pelvis (CAP) was performed and showed a 4.2 × 2.8 cm focus of duodenal thickening and soft tissue prominence, concerning for duodenal neoplasm (Fig. 1). The stomach appeared fluid-filled and dilated, suggesting a component of outlet obstruction. A 2.4 × 2.2 cm heterogeneously enhancing soft tissue nodule arising from the inferior aspect of the third portion of the duodenum was noted that may represent direct neoplastic extension versus an abnormal lymph node (Fig. 2). Several enhancing nodular lesions involving the fourth portion of the duodenum and jejunum measuring up to 1.4 cm were also noted. A 3.7 × 2.8 cm right adrenal mass with pre-contrast attention of less than 10 was seen, consistent with an adrenal adenoma (Fig. 3).
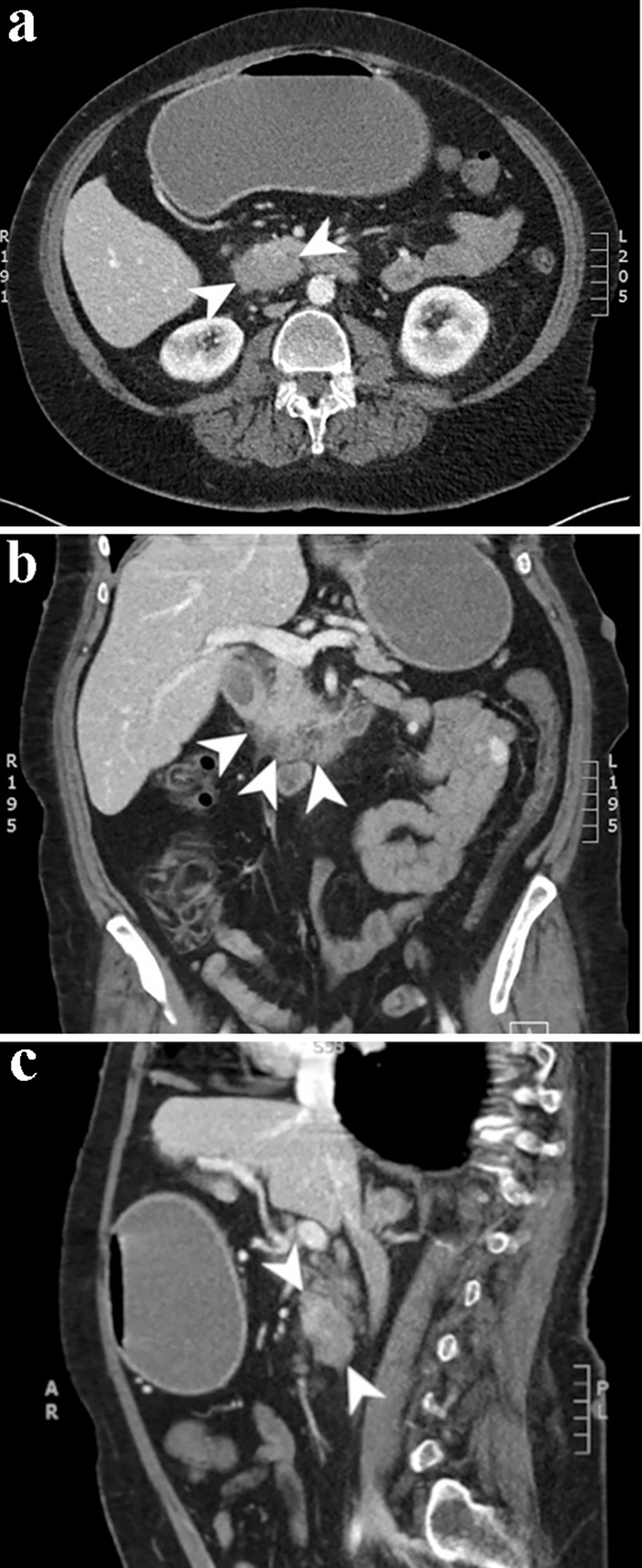 Click for large image | Figure 1. Computed tomography scan of the abdomen and pelvis with and without contrast. Axial (a), coronal (b), and sagittal (c) slices from the venous phase of a computed tomography scan demonstrating a 4.2 × 2.8 cm focus of duodenal wall thickening and soft tissue prominence concerning for a duodenal neoplasm. |
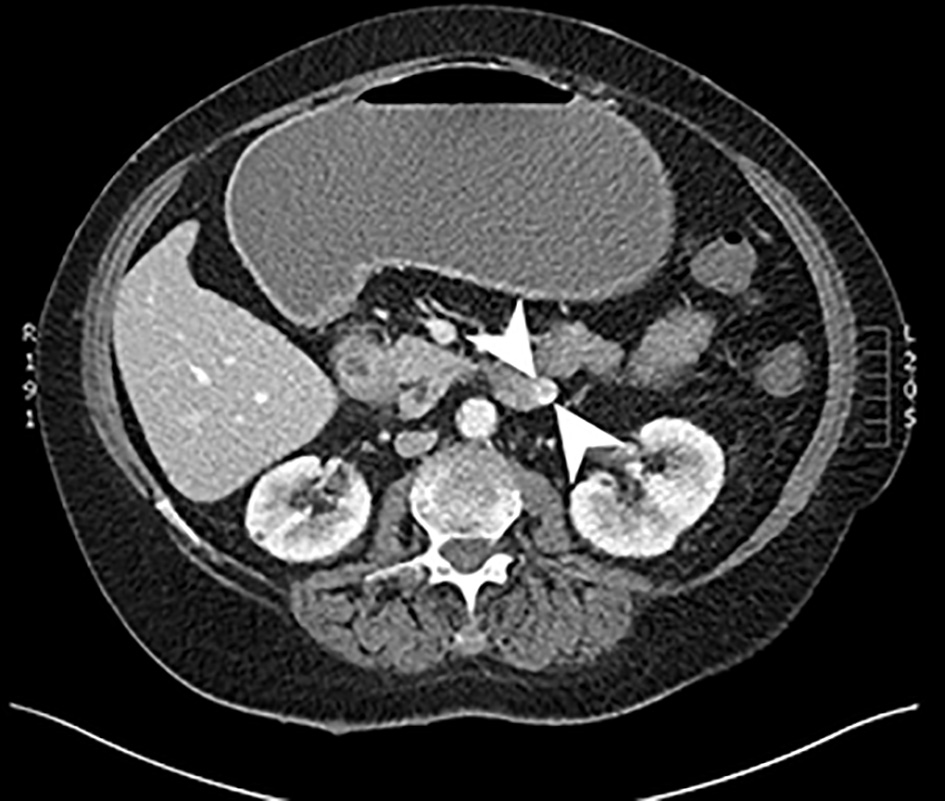 Click for large image | Figure 2. Computed tomography scan of the abdomen with and without contrast. An axial slice from the venous phase of a computed tomography scan showing a 2.4 × 2.2 cm heterogeneously enhancing soft tissue mass arising from the inferior aspect of the third portion of the duodenum. |
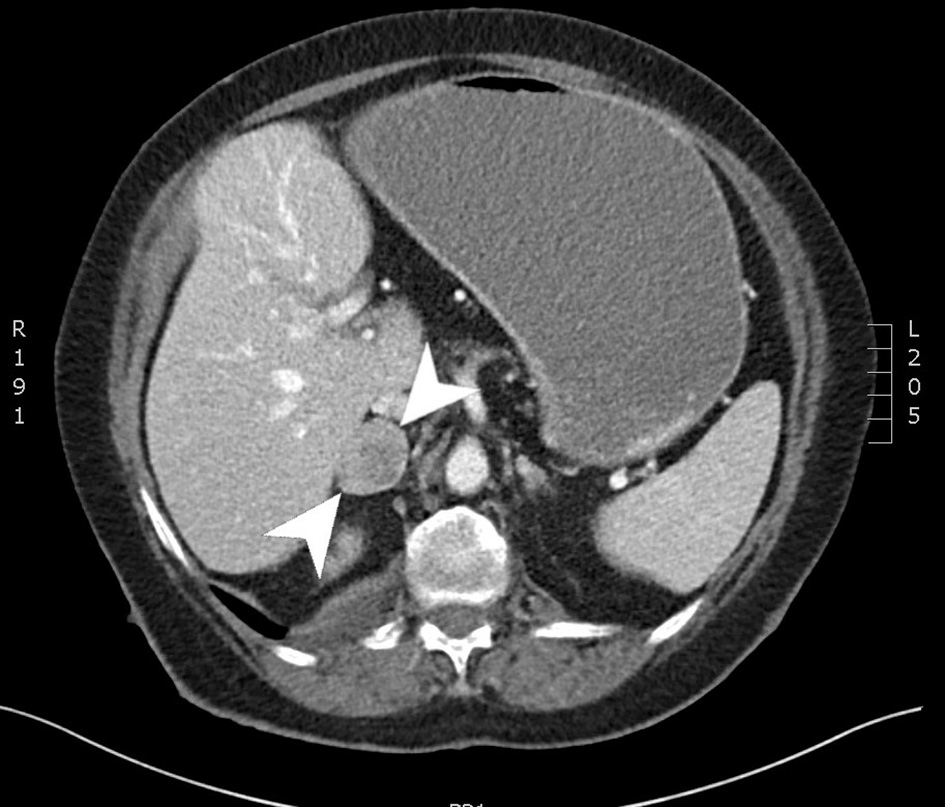 Click for large image | Figure 3. Computed tomography scan of the abdomen with and without contrast. An axial slice from the venous phase of a computed tomography scan showing a 3.7 × 2.8 cm right adrenal mass. |
At this point, the duodenal obstruction raised concern for a duodenal adenocarcinoma. Given the patient’s history of neurofibromas, the right adrenal mass noted on the CT scan was concerning for a pheochromocytoma. Surgical resection was recommended for the patient based on the FNA biopsies that showed malignancy, and her clinical condition. Plasma metanephrines were ordered and were within normal limits, ruling out a pheochromocytoma.
The patient underwent an exploratory laparotomy, right adrenalectomy, pancreaticoduodenectomy, mesenteric nodal dissection, and portal nodal dissection on the second day of admission. The adrenal mass was palpated intra-operatively but did not result in any spikes in blood pressure. On final pathology, she was found to have a 4.7 cm pT3bN2M0R1 peri-ampullary combined poorly differentiated carcinoma and a well-differentiated grade 1 (Ki67 1%) glandular NET with lymphovascular and perineural invasion (Figs. 4 and 5). Three tumor deposits of poorly differentiated carcinoma involving the periduodenal/peripancreatic soft tissue were identified (Fig. 5). The carcinoma and NET components stained positive for chromogranin, CK7, and CDX2 (Fig. 6). Low-grade pancreatic intraepithelial neoplasia (panIN-1) was also noted. One mesenteric lymph node was found to be involved by metastatic combined poorly differentiated carcinoma and well-differentiated grade 1 NET. In addition, seven foci (< 1.7 cm) of GISTs were found in the duodenum with a final pathologic stage of mpT1N1M0R1 and a mitotic rate of < 1 mitosis per 5 mm2 (Fig. 7). The GIST stained positive for KIT (CD117) and CD34 (Fig. 8). The specimen from the right adrenalectomy revealed an adrenal cortical adenoma that stained positive for melan-A and inhibin (Fig. 9). A neurofibroma was resected during the procedure, confirmed to be a neurofibroma on microscopic examination, and stained positive for SOX10 (Fig. 10).
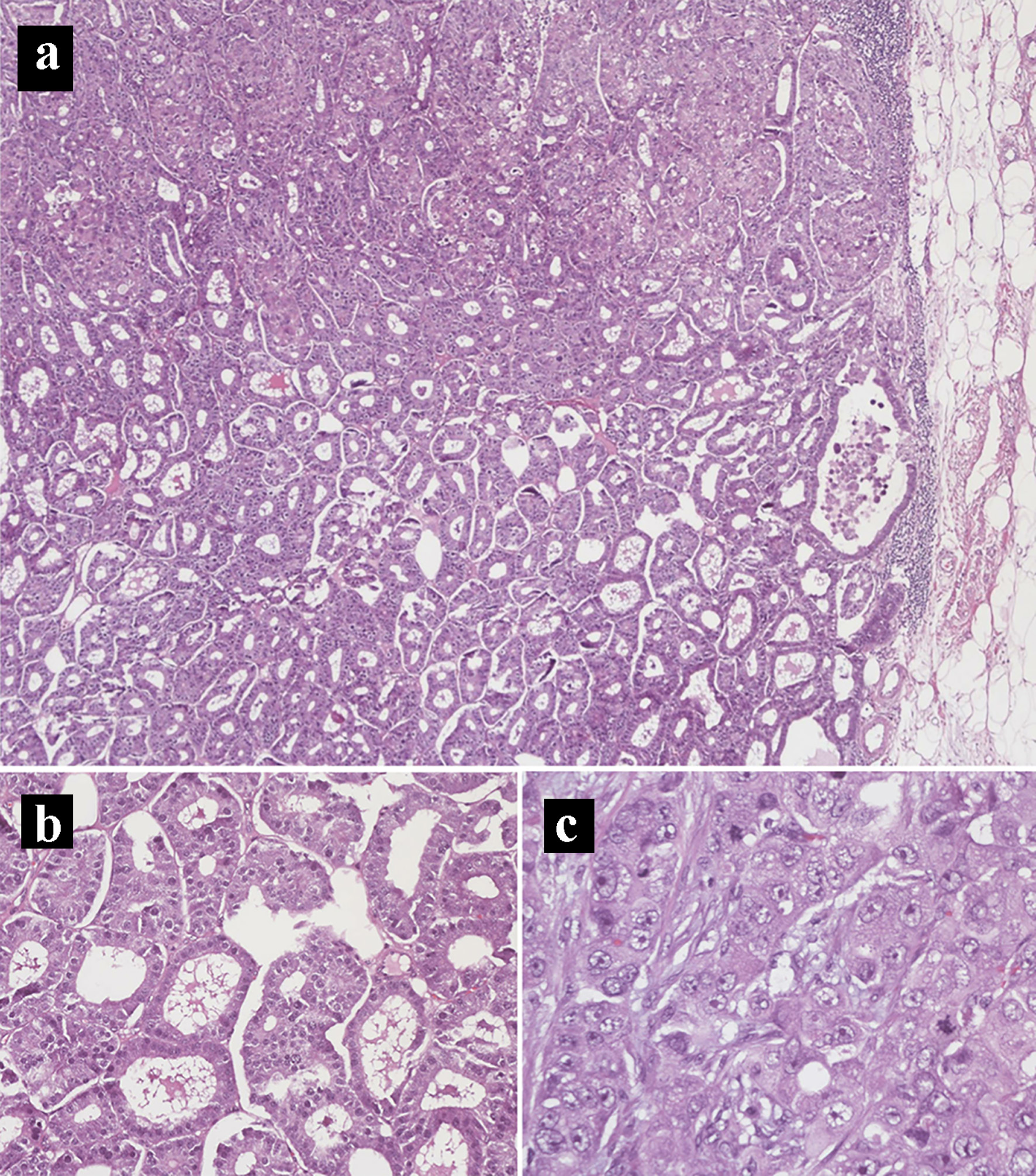 Click for large image | Figure 4. Hematoxylin and eosin histology slides of the primary pancreatic ductal adenocarcinoma and neuroendocrine tumors. (a) Mixed pancreatic ductal adenocarcinoma (top) and well-differentiated neuroendocrine tumor (bottom) (× 40). (b) Neuroendocrine tumor component. (c) Pancreatic ductal adenocarcinoma component in a solid pattern with high-grade nuclei, prominent nucleoli, and frequent mitoses (× 400). |
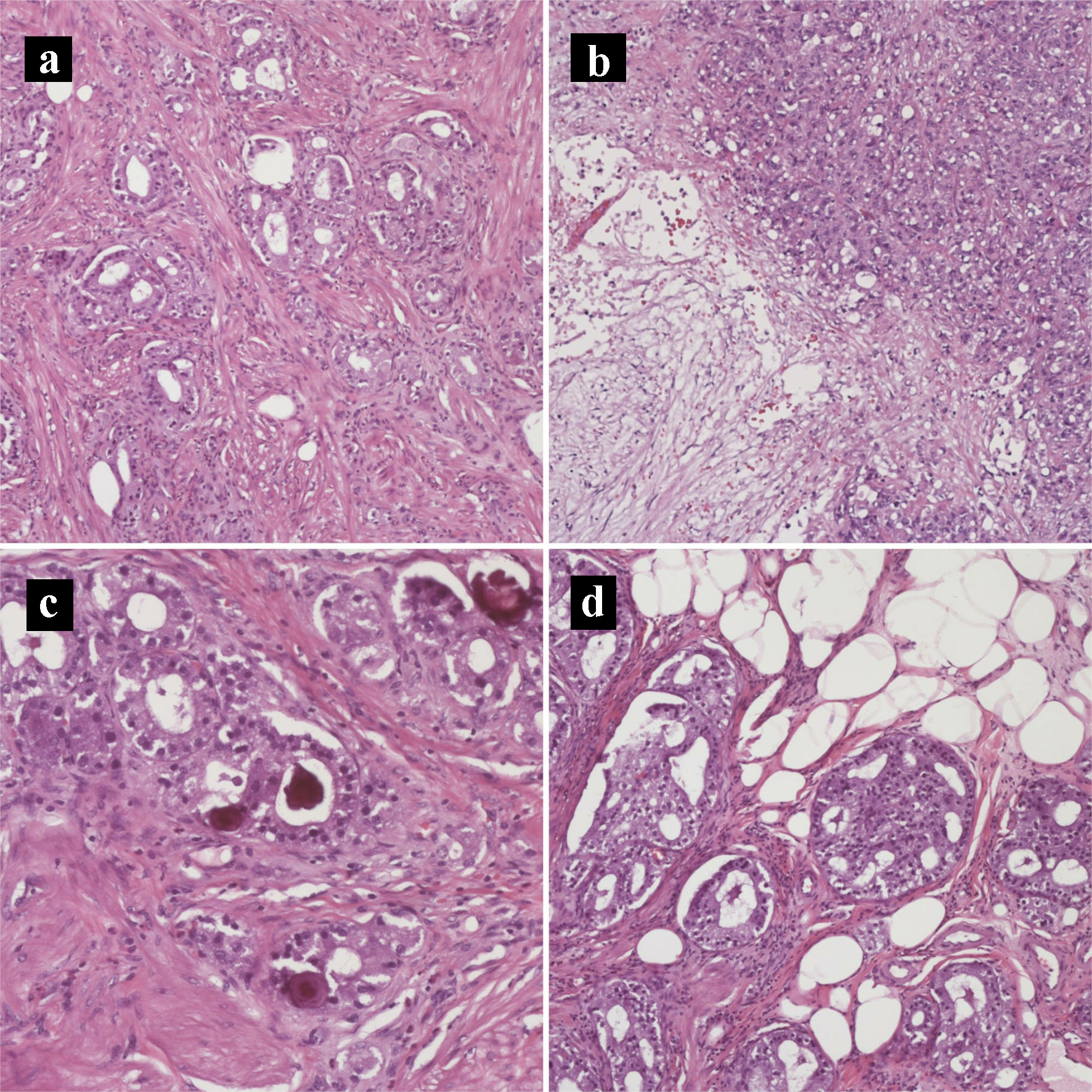 Click for large image | Figure 5. Hematoxylin and eosin histology slides of the primary pancreatic ductal adenocarcinoma and neuroendocrine tumors. (a) Tumor invading the duodenal muscularis propria (× 200). (b) Pancreatic ductal adenocarcinoma with necrosis (× 100). (c) Psammomatous calcifications noted focally (× 200). (d) Tumor invading duodenal fat (× 200). |
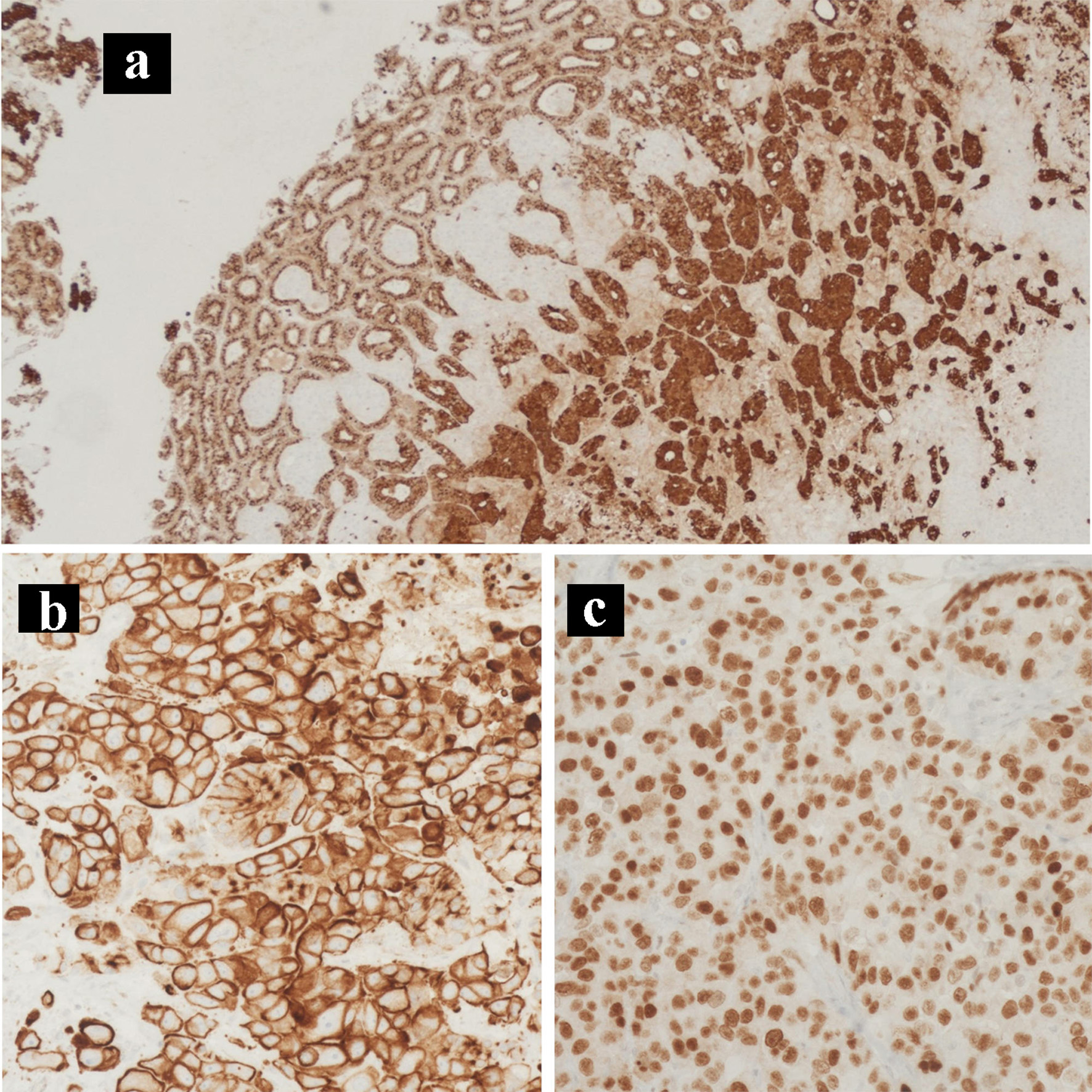 Click for large image | Figure 6. Immunohistochemical staining of the primary tumor. IHC stains of the primary pancreatic adenocarcinoma and neuroendocrine collision tumor. (a) Chromogranin IHC stain (× 100). (b) CK7 IHC stain (× 200). (c) CDX2 IHC stain (× 400). IHC: immunohistochemical. |
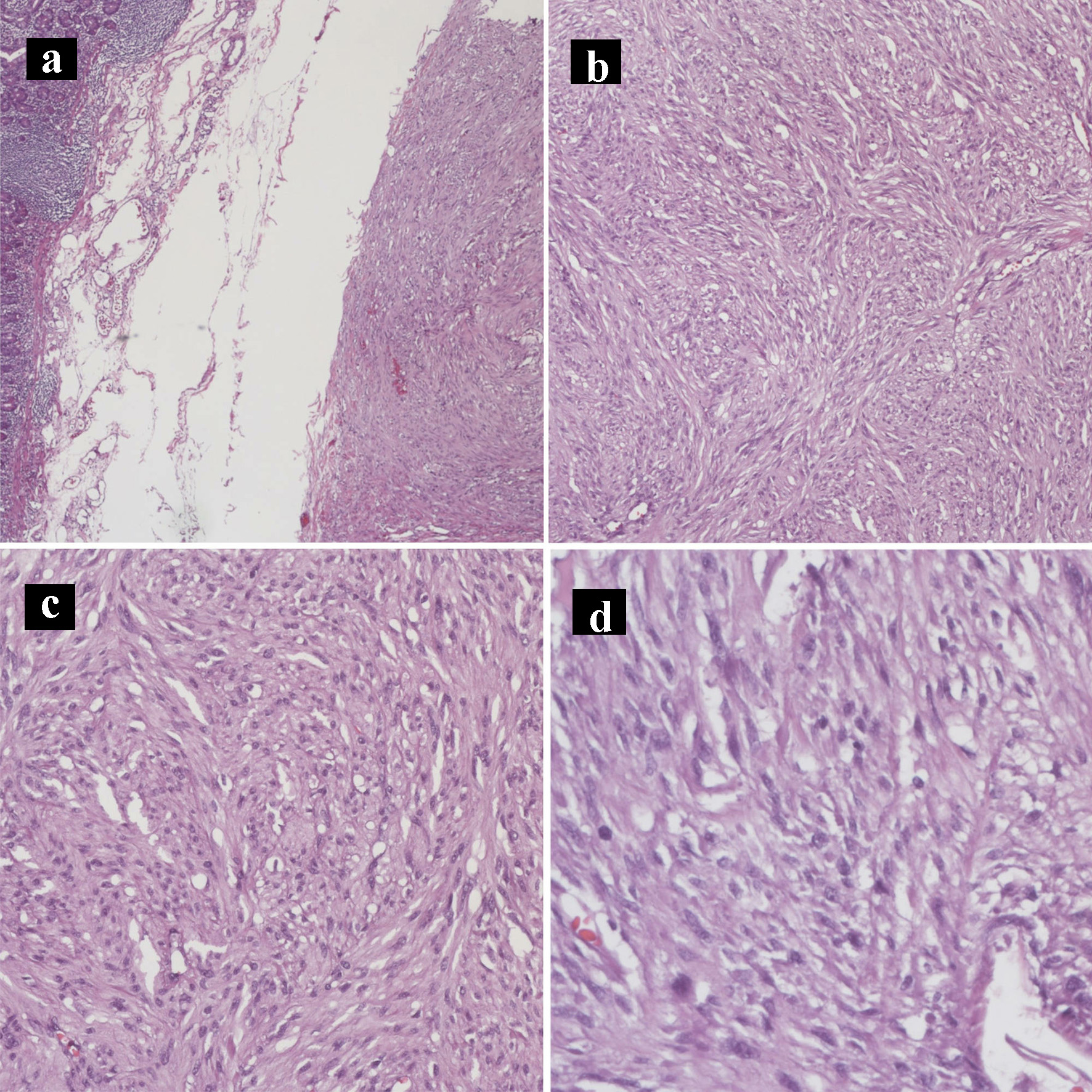 Click for large image | Figure 7. Hematoxylin and eosin histology slides of the gastrointestinal stromal tumor. Gastrointestinal stromal tumor characterized by bland spindle cells with faintly eosinophilic cytoplasm in a syncytial pattern and elongated nuclei with inconspicuous nucleoli (a: × 40; b: × 100; c: × 200; d: × 400). |
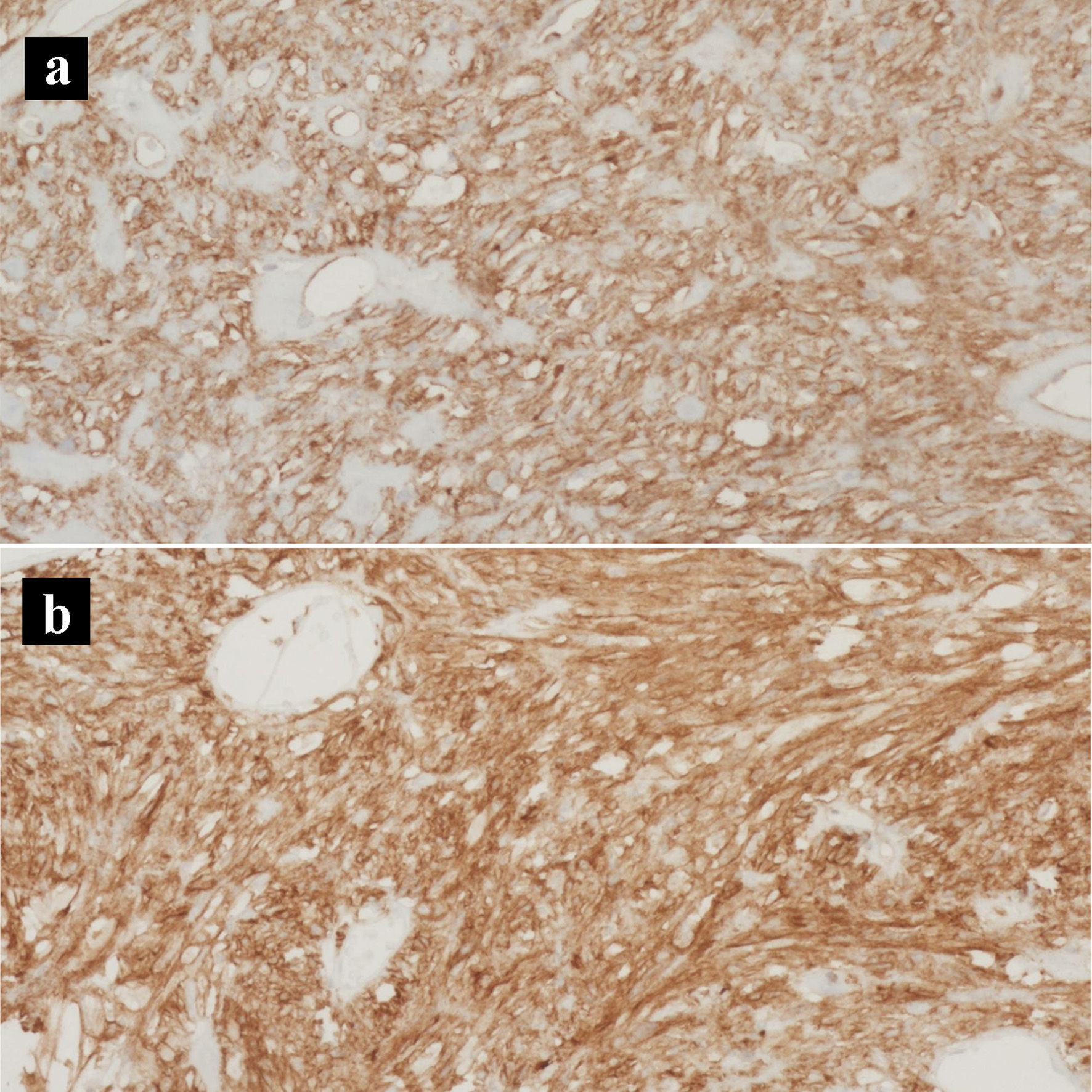 Click for large image | Figure 8. Immunohistochemical stains of the gastrointestinal stromal tumor. (a) CD117 IHC stain (× 200). (b) CD34 IHC stain (× 200). IHC: immunohistochemical. |
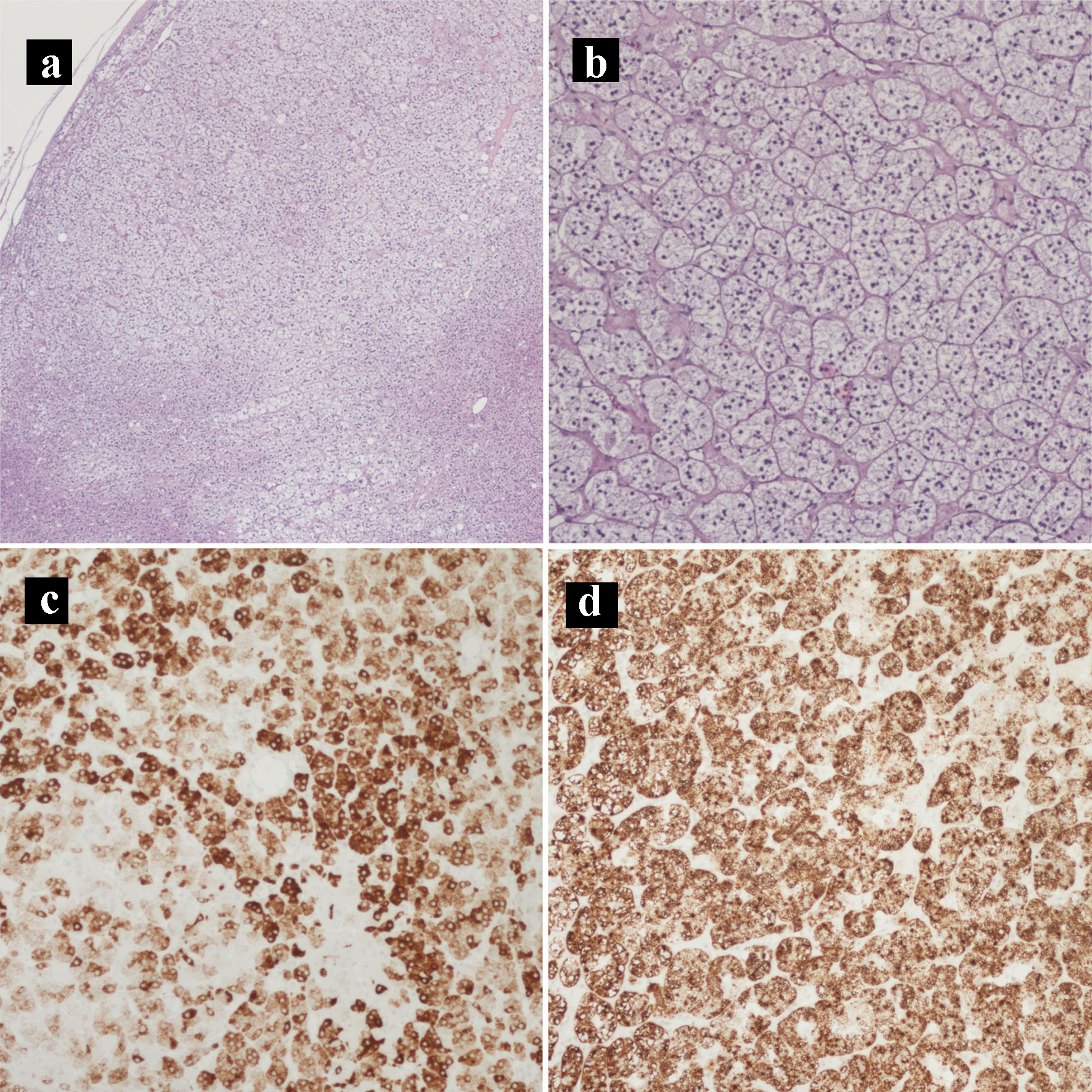 Click for large image | Figure 9. Hematoxylin and eosin histology slides and immunohistochemical stains of the adrenocortical adenoma. (a) Adrenocortical adenoma (× 40). (b) Adrenocortical adenoma with the characteristic clear cells with abundant and finely vacuolated cytoplasm (× 100). (c) MELAN-A IHC stain (× 100). (d) Inhibin IHC stain (× 100). IHC: immunohistochemical. |
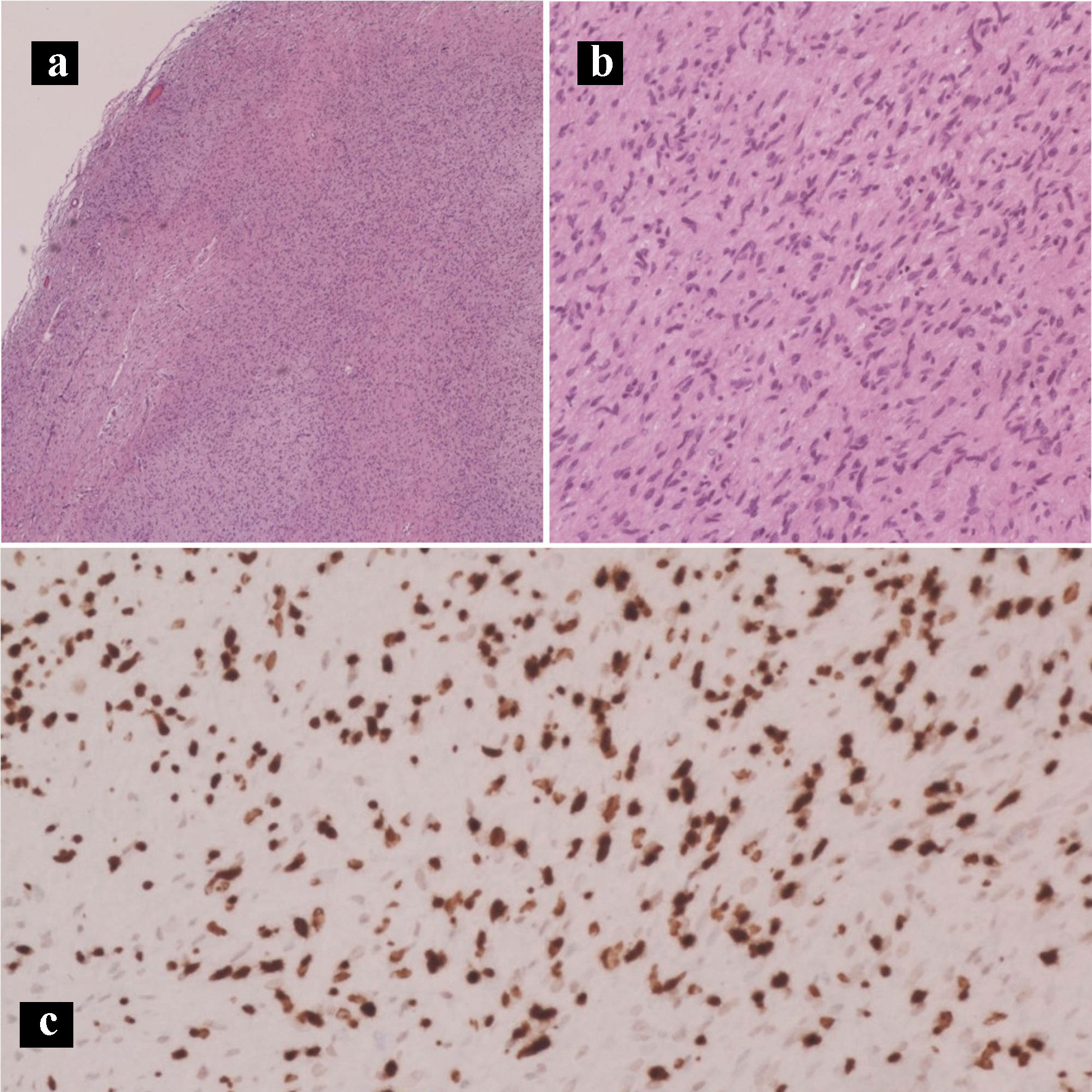 Click for large image | Figure 10. Hematoxylin and eosin histology slides and immunohistochemical stains of the neurofibroma. Neurofibroma characterized by Schwann cells with wire-like collagen fibrils and wavy serpentine nuclei with pointed ends (a: × 40; b: × 200). (c) SOX10 IHC stain (× 100). IHC: immunohistochemical. |
She tolerated the procedure and did well in the immediate post-operative period, apart from a grade A pancreatic fistula. The patient was then seen by oncology and was started on gemcitabine and capecitabine. Unfortunately, she developed metastatic disease 8 months post-operatively and was placed on hospice.
| Discussion | ▴Top |
We present a rare case of an adenoneuroendocrine pancreatic collision tumor with incidental GIST in a patient with neurofibromatosis type 1 (NF-1). The exact pathologic mechanism of collision tumors is unknown, but there are four main theories in the literature [3]. One is neoplastic heterogenicity, where two different neoplastic cells occur in the same area by chance. Second is cancerization theory, in which areas that have recurrent damage or high rate of turnover will have increased chance of developing separate neoplasms. Third is the interaction theory, which suggests that one neoplasm produces changes that induce an environment conducive to a second independent neoplasm. Fourth is that there is neoplastic heterogenicity during the formation of a neoplastic cell that results in dedifferentiation [3].
Imaging may reveal two separate components, heterogeneity, or abnormal accumulation in fluorodeoxyglucose-positron emission tomography (PET) [4], but alone may not be sufficient to diagnose these tumors. Biopsies also cannot sample the entirety of the tumor [4]. Thus, it is crucial to keep this differential in mind. Their presence significantly alters the biology of the tumor, and treatment is dependent on proper diagnosis. In the literature, collision tumors are most commonly found in the crania, lung, gastroesophageal junction, liver, rectum, bladder, and uterus, with the vast majority (96.2%) of tumors consisting of only two distinct components [5]. MANECs are very rare, with only 0.23 cases per 1,000,000 individuals reported in 2000, and 1.16 cases per 1,000,000 individuals reported in 2016 [6].
Furthermore, collision tumors are not easy to morphologically distinguish from composite tumors, in which two types of tumors are in close proximity with actual histologic merging of the different tumor cells [7]. In more recent studies, immunohistochemistry has been a method utilized to help distinguish between the two tumors [7], but the diagnosis is usually not obtained until after surgical resection.
Some studies recommend treating the more aggressive element, and thus the chemotherapeutic and radiation regimens are typically followed for PDAC in a collision tumor consisting of PDAC and a less aggressive tumor. However, due to a small number of reported cases, clinical behavior or MANECs of the pancreas is still unclear and a standardized treatment protocol has not been established.
Wang et al presented a case of a 51-year-old female patient who had a 7-month history of progressively worsening right upper quadrant pain with normal bilirubin, lipase, CA19-9, and immunoglobulin G subclass 4 levels. Preoperative diagnosis was clinically challenging as radiology and fine-needle biopsy were consistent with a typical neuroendocrine mass. Upon final pathology, however, there was a focus of ductal adenocarcinoma without a transition zone [8]. This highlights the difficulty of preoperative diagnosis especially in light of normal serum markers that are typically found in adenocarcinoma. Additionally, special attention should be paid to atypical radiologic features that may suggest a collision tumor.
Serafini et al presented an interesting case of a collision tumor consisting of pancreatic ductal adenocarcinoma involving the whole pancreas as well as a metastatic NET, misinterpreted as a malignant intraductal papillary mucinous neoplasm (IPMN); incidentally, a GIST located in the duodenal peripancreatic wall and the head of the pancreas was also found at operation [9].
In a recent systematic review, Frizziero et al reviewed 687 publications regarding mixed neuroendocrine and non-neuroendocrine collision tumors, in which 91 studies with 2,427 patients were included. The pancreas was the site of origin in 3.7% (n = 90) of cases, in which 92.2% of the non-neuroendocrine component was PDAC [10]. Surgery was the treatment of choice in nearly all potentially curable cases; however, median overall survival was poor at 12 - 18 months [10].
Another case series reported a median survival of 10 months for pancreatic and peri-ampullary collision tumors even after radical resection, which was significantly lower than 27 months in patients with PDAC who received radical surgery and with patients who received a palliative operation [11]. This speaks to the potentially more aggressive nature of collision tumors, which may or may not be due to the difficulty in their diagnosis. PDAC classically has a 5-year survival rate of 5-10%, whereas 5-year survival rate for MANEC collision tumors has been 0% in the literature thus far [12]. Thus, collision cancers of the pancreas and periampullary region are difficult to diagnose and prognosis is poor even after radical resection and adjuvant chemotherapy. This demonstrates a dire need for improved diagnostics and treatment course for collision tumors of the pancreas.
Our patient had a history of NF-1 and also presented with an incidentally found GIST, which contributed to the complexity of her case presentation. NF is an autosomal dominant disorder caused by the inactivation of the NF-1 gene. The frequency of intra-abdominal manifestations in NF-1 varies greatly, ranging from 5% to 25% in studies, but GIST is considered the most common [13]. The molecular pathogenesis of epithelial and other gastrointestinal neoplasms of NF-1 patients remains unknown, but NF-1 associated GISTs appear to have a loss of heterozygosity at 14q and 22q, which may contribute to the relatively early phase of tumor development [13]. In a systematic review, Salvi et al found that NF-1 associated GISTs seem to have a distinct phenotype, specifically they contained multiple tumors, were small in diameter, mostly indolent, and had absence of KIT/PDGRFA mutations [14]. This may explain why they are most often incidentally found.
Limitations include the inherent nature of a case report, the poor prognosis of MANECs and thus little time for collection of clinical data, as well as the fact that our patient had comorbidities that prevented her from receiving FOLFIRINOX, which is the standard first-line treatment for PDAC. Our case reported a rare adenoneuroendocrine pancreatic collision tumor with high malignant potential. Further investigation of more of these rare cases is necessary to determine its clinical behavior and the best course of treatment.
Conclusion
We report a rare case of a pancreatic collision tumor consisting of both pancreatic adenoneuroendocrine carcinoma and incidental GIST. Collision tumors and specifically MANECs of the pancreas are extremely rare and clinical behavior is unclear, and thus no standardized treatment exists. It is important to keep this differential in mind as preoperative diagnostics do not reliably identify these tumors, and treatment as well as prognosis may vary dependent on the tumor characteristics.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Subject consent has been waived under the IRB approved study protocol and the identity of the subject has been protected.
Author Contributions
CC, MBD, and WK performed data collection, manuscript writing, critical revision of the manuscript, and final approval. PJM, ALB, EC, HO, and DRJ performed manuscript writing, critical revision of the manuscript, and final approval.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Imaoka K, Fukuda S, Tazawa H, Kuga Y, Mochizuki T, Hirata Y, Fujisaki S, et al. A mixed adenoneuroendocrine carcinoma of the pancreas: a case report. Surg Case Rep. 2016;2(1):133.
doi pubmed - Liu Y, Wang C, Hu X, Wang M, Wang Y, Ye M, Liu Y. Concurrent ductal adenocarcinoma, pseudocyst, and neuroendocrine tumor of the pancreas: A case report. Medicine (Baltimore). 2020;99(30):e21354.
doi pubmed - Bulte CA, Hoegler KM, Khachemoune A. Collision tumors: A review of their types, pathogenesis, and diagnostic challenges. Dermatol Ther. 2020;33(6):e14236.
doi pubmed - Sung CT, Shetty A, Menias CO, Houshyar R, Chatterjee S, Lee TK, Tung P, et al. Collision and composite tumors; radiologic and pathologic correlation. Abdom Radiol (NY). 2017;42(12):2909-2926.
doi pubmed - Schizas D, Katsaros I, Michalinos A, Damaskos C, Garmpis N, Ntomi V, Agrogiannis G, et al. Collision tumors of the gastrointestinal tract: a systematic review of the literature. Anticancer Res. 2018;38(11):6047-6057.
doi pubmed - Wang J, He A, Feng Q, Hou P, Wu J, Huang Z, Xiao Z, et al. Gastrointestinal mixed adenoneuroendocrine carcinoma: a population level analysis of epidemiological trends. J Transl Med. 2020;18(1):128.
doi pubmed - Miyamoto R, Kikuchi K, Uchida A, Ozawa M, Kemmochi A, Sano N, Tadano S, et al. Collision tumor consisting of a colorectal adenocarcinoma and dissemination of a gastric adenocarcinoma. SAGE Open Med Case Rep. 2018;6:2050313X17751839.
doi pubmed - Wang Y, Gandhi S, Basu A, Ijeli A, Kovarik P, Sekosan M, Demetria M. Pancreatic collision tumor of ductal adenocarcinoma and neuroendocrine tumor. ACG Case Rep J. 2018;5:e39.
doi pubmed - Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Merigliano S, Sperti C. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: a case report and review of the literature. World J Surg Oncol. 2017;15(1):93.
doi pubmed - Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed neuroendocrine non-neuroendocrine neoplasms: a systematic review of a controversial and underestimated diagnosis. J Clin Med. 2020;9(1):273.
doi pubmed - Niu GM, Jin DY, Ji Y, Hou J, Wang DS, Lou WH. Survival analysis of pancreatic and periampullary collision cancers. J Dig Dis. 2010;11(4):231-236.
doi pubmed - Pihlak R, Weaver JMJ, Valle JW, McNamara MG. Advances in molecular profiling and categorisation of pancreatic adenocarcinoma and the implications for therapy. Cancers (Basel). 2018;10(1):17.
doi pubmed - Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen's disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5(9):852-862.
- Salvi PF, Lorenzon L, Caterino S, Antolino L, Antonelli MS, Balducci G. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol. 2013;2013:398570.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
