| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Original Article
Volume 12, Number 2, December 2022, pages 29-37
Cost Analysis of a Fracture Liaison Service: A Prospective Study for Secondary Prevention After Fractures of the Hip
Gershon Zingera, b , Amit Davidsona, Noa Sylvetskya, Yedin Levya, Amos Peysera
aDepartment of Orthopedic Surgery, Shaare Zedek Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 9103102, Israel
bCorresponding Author: Gershon Zinger, Department of Orthopedic Surgery, Shaare Zedek Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 9103102, Israel
Manuscript submitted June 9, 2022, accepted July 14, 2022, published online December 30, 2022
Short title: Cost Analysis of an FLS Service
doi: https://doi.org/10.14740/jcs460
| Abstract | ▴Top |
Background: Fracture liaison services (FLSs) have proven to be effective in treating osteoporosis associated with fragility fractures. For patients with fragility fractures of the hip, FLS programs are expected to be cost-effective because of the high risk of re-fracture and the high cost of fracture treatment. In this study, we evaluated the essential factors in determining whether the FLS saves or loses more than it costs.
Methods: A prospective-randomized study was done in patients with hip fragility fractures using a hospital-based FLS program in parallel with a cost analysis. Data were generated from a cohort of patients using actual data for FLS effectiveness, individual costs of hip fracture treatment, and medication costs based on an accepted treatment algorithm.
Results: There were 200 patients randomized and 180 analyzed for costs. Results showed that the cost-benefit of the FLS was dependent on the medication used for osteoporosis. Specifically, using the medication algorithm in this study, the loss per patient enrolled in the FLS was $671 for a 2-year period. If intravenous zoledronic acid had been used, then the loss would have been $221. If only oral bisphosphonates had been used, then the FLS would have saved $109 per patient for a 2-year period.
Conclusions: The analysis done here shows that medication cost is the critical component in cost-effectiveness of an FLS program. Additional work needs to be done refining the medication algorithm considering medication costs but individualized to patient needs based on fracture risk.
Keywords: Osteoporosis; FLS; Fracture liaison; Cost analysis; Secondary prevention
| Introduction | ▴Top |
Osteoporosis is a worldwide epidemic. Despite well-known protocols, less than 15% of patients get treated for underlying osteoporosis after a hip fracture [1]. For those who sustain a fragility fracture, the risk of a subsequent fracture is 2 - 4 times higher [2, 3]. Strategies to improve compliance with recommended standards have generally not been effective [4]. The exceptions are those programs with a fracture liaison service (FLS) with a dedicated team [5]. FLS programs appear to be the best and potentially only reliable method to get patient started on osteoporosis treatment. The challenge then is who is willing to pay?
The purpose of this study was to better understand the factors that influence the costs and potential savings of an FLS program within a national healthcare network. Patients who suffer a hip fragility fracture are at a high risk for secondary fragility fractures. This high risk of re-fracture along with the high cost of treating additional fractures makes this post hip fracture group uniquely suited as a focus for secondary prevention. The costs of a secondary prevention program may be offset by the savings of preventing future expected fragility fractures. The International Osteoporosis Foundation (IOF) recommends that programs interested in starting a FLS program should start with secondary prevention of hip fractures for precisely these reasons.
We performed a prospective randomized study with a dual purpose of improving compliance with starting osteoporosis medication after hip fragility fractures in parallel with an analysis of the costs incurred for treating these fractures. Specifically, we calculated the actual costs of hip fracture treatment for each patient individually. Using these calculated costs with the actual effectiveness and costs of our FLS, we were able to accurately estimate the costs and savings of having a secondary FLS program. The objective of this study was to elucidate the factors that can make a secondary FLS program either profitable or a burden to a system. In addition, we evaluated the costs and savings separately from the perspective of the hospital and the insurance system to determine whether an FLS would be beneficial for one or the other.
| Materials and Methods | ▴Top |
Inclusion/exclusion criteria
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (clinical trials number: 201497CTIL). All patients over the age of 50 admitted with a fragility-related hip fracture were considered for inclusion. A fragility fracture is defined here as a fracture resulting from a low energy fall typically occurring while standing or walking. Our facility is a level I trauma center, one of two hospitals serving a population of approximately 900,000. Hip fractures included those in the subcapital, femoral neck, intertrochanteric or subtrochanteric region. Fractures of the trochanter alone, those involving the shaft or peri-prosthetic region were not included. Exclusion criteria also included patients with a fracture sustained in a non-low energy fall, those with metastatic cancer or known metabolic bone disease or patients in end-of-life care. Patients who were discharged to another facility before their surgery were also excluded. Patients unable to undergo consent because of dementia were excluded, but if their dementia was mild and consent could be obtained, they were included.
Data management
Data were entered into the database created using the Research Electronic Data Capture (RedCAP) from Vanderbilt University and available through the research department provided by our medical center.
Randomization
All patients that met the study criteria gave written informed consent before randomization. The patients were prospectively randomized equally into one of two levels of intervention (see below). The statistician used a random number generator for one of two options (group A or B) using 2 blocks of 100. The sequence was not concealed from the research assistant who enrolled the patients into the study. The study was not blinded for the intervention.
Intervention
The control group received a letter at time of discharge encouraging their primary care physician to start medication for osteoporosis. The intervention group had four interventions including printed information about osteoporosis, a dual energy X-ray absorptiometry (DEXA) scan, a specific treatment recommendation (Fig. 1) to give to their primary care physician to initiate treatment, and monthly phone calls from the study coordinator for 4 months.
 Click for large image | Figure 1. Medication treatment algorithm with recommendation. |
Treatment algorithm
The treatment algorithm was designed to guide primary care physicians on medication treatment for secondary prevention. It was designed to give them two options for each of three patient scenarios. The default treatment recommendation was intravenous bisphosphonates based on the success of the Horizon study [6] and reduced need for patient compliance compared to weekly oral bisphosphonates. Denosumab was an option for patients who were considered “treatment failures” if they had a fragility fracture while on bisphosphonates for 2 years of more. Other reasons for recommending denosumab included patients who had renal failure, declined intravenous zoledronic acid because of previous experience or concern about flu-like symptoms or patients with mobility issues that were not able or willing to come to the clinic for an infusion. Teriparatide was an option for patients who were treatment failures on bisphosphonates or had a higher risk of additional secondary fractures based on the individual patient profile that included DEXA and fracture risk assessment tool (FRAX) scores.
Cost versus benefit analysis
To determine the cost versus benefit, the costs and savings need to be annualized, so that there is a common denominator. Then costs and savings of our FLS program were determined along with the expected savings for a 2-year period. This is further elucidated below.
Savings of the FLS program
The savings of an FLS program is the amount saved by reducing the costs of having to treat future secondary fractures. Specifically, there are three related components. The first component is the effectiveness of an FLS program in getting patients on recommended treatment. The second component is the effectiveness of that treatment. That is, how much will appropriate treatment reduce the frequency of secondary fractures compared to no treatment. The third component is the costs and frequency of the different types of additional fragility fractures. Then, knowing the costs of treating secondary fractures, and knowing the reduction in these fractures, the amount saved can be calculated.
For example, if treating a hip fracture cost $100 and there are normally 20 fractures in a 2-year period, then costs would be $2,000 over 2 years treating hip fractures. If the FLS program reduces these fractures by 10% compared to no treatment, then the savings would be $200 over 2 years. However, if only 50% of patients enrolled in the FLS program and started the medication, then the savings would be $100 over 2 years. One could then compare this to the costs of an FLS program to determine if money was overall saved or lost.
Costs of fragility fracture treatment
For the cost of treating hip fractures, we used our prospective data calculated for this study. For other fragility fractures such as spine, and distal radius or proximal humerus (non-hip and non-vertebral), we calculated the costs for the type of fracture including an estimate of how often they are treated conservatively versus surgically and the different costs for treatment (Supplementary Material 1, www.currentsurgery.org).
System costs of treating hip fractures
When a patient is hospitalized for a hip fracture, the hospital gets reimbursed for part of their costs from the national health insurance. Unfortunately for the hospital, the reimbursement for many procedures is not sufficient to cover the hospital costs of care. The hospital loses money taking care of most hip fractures.
From the perspective of the insurance system, the payment to the hospital is a loss. Their costs are reimbursed by a combination of monthly fees and government support. However, for hip fractures, their costs also exceed their reimbursement. In the end, the burden of care falls to the population through either monthly fees and/or taxes. This is represented as cost to the system and is determined by the combined loss to both the hospital and the national insurance.
Hospital perspective for hip fracture care
The costs and reimbursement to each patient hospitalized for hip fractures were individually calculated and a detailed analysis was performed. The costs for hip fracture care were impacted by many factors including for example preoperative evaluation, the type of implant and the length of hospitalization. Costs related to complications or re-admission within 30 days were an important part of the analysis. If the patient was admitted more than 30 days after the initial surgery, it was not considered as part of the cost of the index procedure. In addition, the costs to the health insurance system, but not to the hospital, for hip fracture care included postoperative rehabilitation whether at a facility or at home.
The main factor affecting reimbursement to the hospital is the length of hospitalization. All hip fractures carry the same diagnosis-related group (DRG). The agreement between the hospital system and the insurance providers is that hospitals are reimbursed by the DRG amount if the patient is discharged within 11 days. If the patient is hospitalized 12 days or longer, the hospital is reimbursed on a per-day basis.
Costs of the FLS program
The cost of an FLS can be determined by the combination of the cost of the FLS coordinator and system overhead as well as the costs of additional medication prescribed for secondary prevention. Each of these components is enumerated below.
Costs to the system for secondary fracture treatment
The Horizon study [6] evaluated the effectiveness in reduction of secondary fractures for three categories including hip, vertebral and non-hip/non-vertebral. The costs for hip fracture treatment were directly calculated as described above. The costs and frequency for vertebral and non-hip/non-vertebral based were estimated based on historical data (Supplementary Material 1, www.currentsurgery.org).
Expected reduction in re-fractures based on Horizon study
To determine the effectiveness of an FLS program on reducing fracture incidence, we used information from the Horizon study [6]. That industry-sponsored study was prospective, well-powered with nearly 2-year follow-up. They used intravenous bisphosphonates that eliminated the issue of compliance. They found refracture rates (of secondary fractures) for those on treatment compared to those not on treatment of 8.6% versus 13.9% respectively. We used their reduced re-fracture rate along with our FLS effectiveness rate and the losses for each fracture type to calculate the savings per patient for each of the three categories of fractures, hip, vertebral and non-hip/non-vertebral (available upon request). Hip fractures were the most expensive for the system and reducing these fractures represented the greatest savings. For non-hip fractures, the hospital profited, and the insurance system lost.
FLS costs
The costs of an FLS have three primary components: the cost of the program coordinator and overhead, the costs of additional laboratory (and DEXA) evaluation and the costs for the medications that the patients take for osteoporosis treatment. The total of these costs was then averaged to provide the cost for FLS care per patient over a 2-year period.
We estimated the FLS coordinator time required for intervention based on a sample of patients, then averaged the time over all patients. Labs that are routinely done on admission for hip fracture care were not considered an added cost for an FLS since they would be needed in any case for preoperative evaluation. Other labs that were indicated based on the FLS program algorithm and not routine were added as FLS costs. For example, parathyroid hormone (PTH) levels had specific indications (calcium level more than 10.5 mg/dL) and this lab when indicated, was included as an added cost. Although the DEXA scan is not required to initiate treatment since a fragility fracture is determined by the mechanism of low energy, DEXA scan is generally recommended and often useful. One advantage of having a baseline DEXA is to follow effectiveness of treatment. Another advantage was in identifying patients with unusually low bone densities that are at higher risk and therefore warrant treatment with more effective and expensive medications [7].
Since the information from the Horizon study [6] was used to calculate reduction of fractures over a 2-year period, we calculated all FLS costs over 2 years to compare it to savings over the same time frame. FLS non-medication costs were then allocated to either the hospital or the national insurance.
| Results | ▴Top |
The enrollment period was from February 21, 2017, to September 15, 2018 when 200 patients were reached. This number was chosen based on a power calculation used for the intervention part of the study [8]. The intervention part of the study prospectively evaluated the effectiveness of our FLS compared to a control group (the intervention program is described below). The cost analysis presented here used prospective data from both groups. Since there is no control group for the cost analysis, no a priori power calculation was needed. During the enrollment period, there were 618 low-energy hip fractures, of which 305 were eligible for enrollment (Fig. 2). Most of the patients not eligible were excluded due to moderate or severe dementia, an exclusion criterion for this study. Sixty-six percent of those eligible agreed to participate in the study. Baseline characteristics were not different between groups (Table 1). The age range was 51 to 95 years with an average age of 79.2 (± 9.2) years. Seventy-two percent were female.
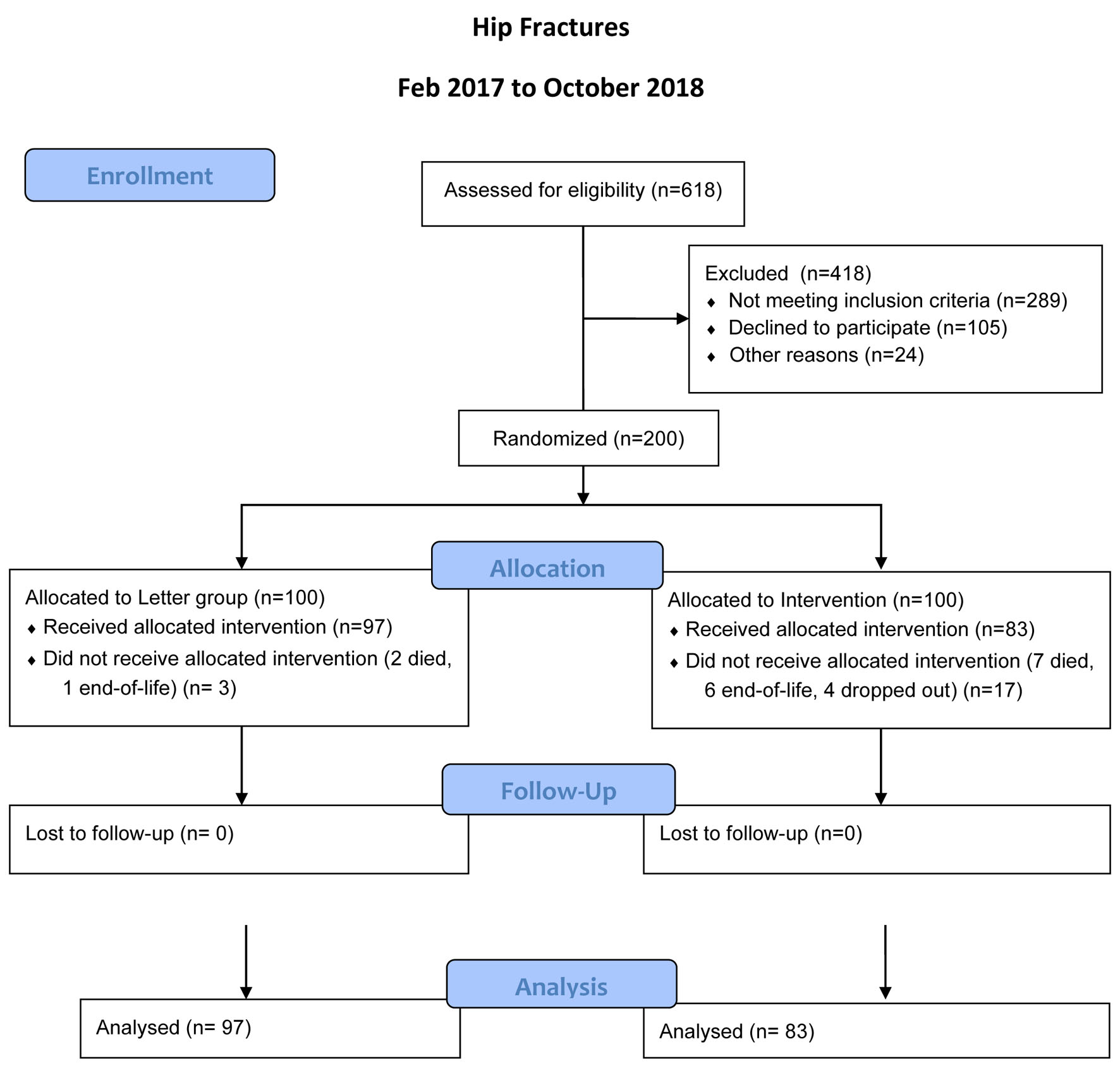 Click for large image | Figure 2. Flow diagram for patient enrollment. |
 Click to view | Table 1. Baseline characteristics of Letter Versus Intervention Groups |
Patients were hospitalized an average of 11.7 days (standard deviation (SD): 14.6) with range of 3 to 133 days (median 8.0 days). This included complications both medical and surgical that resulted in prolonged hospitalizations.
Preoperative hip imaging (in addition to plain X-rays) was done in 19 patients including 17 that required a computed tomography (CT) scan and two that required an magnetic resonance imaging (MRI) to confirm non-displaced fractures after plain X-rays could not confirm the diagnosis. Preoperative imaging was also done for medical reasons including echocardiogram in 14 and head CT in 18.
Hip implants included cannulated screws in 25 (12.5%), gamma nail in 97 (48.5%), bipolar in 38 (19%), total hip arthroplasty (THA) in 3 (1.5%), PF2 (Zimmer implant) in 16 (8%), percutaneous compression plating (PCCP) in 13 (6.5%), proximal femoral nail anti-rotation (PFNA) in 5 (2.5%), and no surgery in 3 (1.5%).
Complications
Overall, there were 40 patients (20%) with complications including 12 (6%) with surgical complications, 22 (11%) with medical complications, and 6 (3%) with both surgical and medical complications.
Surgical complications included deep wound infection (7), superficial wound infection (3), hardware failure (4) and artery perforation or pseudoaneurysm requiring interventional radiology coiling (2).
Medical complications (some with more than one complication in a patient) included pulmonary embolism (1), urinary tract infection (5), renal failure (12), stroke (1), heart failure exacerbation (12), pneumonia (7), other (6) including gallstones, atrial fibrillation, gastrointestinal (GI) bleed, intra-cranial bleed, terminal ileitis, general deterioration, and death (6).
Hospital perspective - cost of treating hip fracture
The average cost per patient of treating a hip fracture for the hospital (including complications) averaged $14,200 (SD $12,850 and range from $2,770 to $109,700) (Fig. 3). Health insurance reimbursement was an average of $10,070 (SD $8,157 and range from $1,999 to $76,220) leaving an average loss for the hospital of $4,132 (SD $5,789 with range of loss of $33,520 to profit of $5,873) per hip fracture.
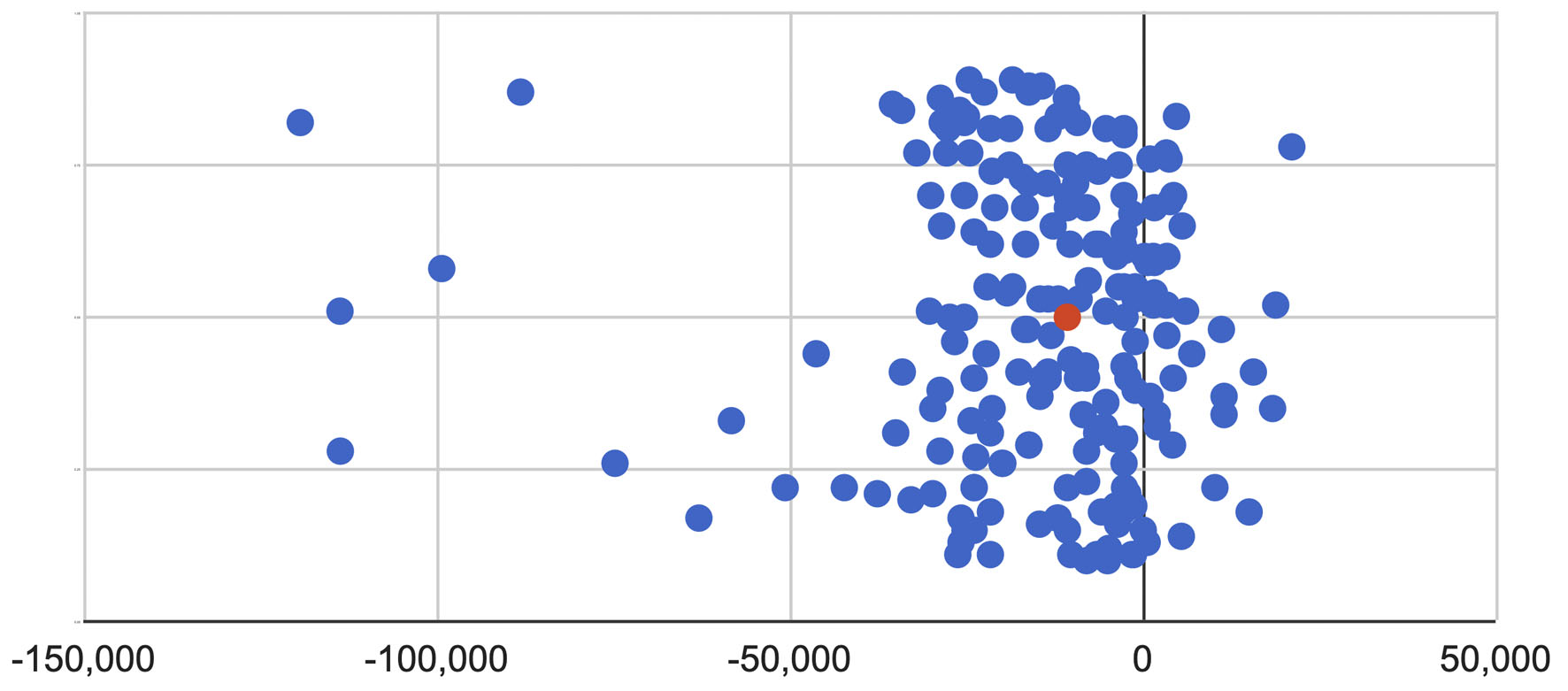 Click for large image | Figure 3. Net gain or loss for each patient from hospital perspective (NIS). |
Net gain or loss in NIS (Israeli Shekels) for each patient (costs in Israeli Shekels were converted to US dollars at the average 2019 rate of 1 USD = 3.5645 Shekel from www.boi.org.il)
National Health Insurance Perspective - cost of treating hip fracture
The average payment from the health insurance to the hospital was $10,070 per hip fracture. Rehabilitation adds approximately $4,619 bringing the total average cost per hip fracture for the health insurance to $14,670.
Costs to the system for hip fracture treatment
The cost to the system includes the combined loss to both the hospital and the health insurance. In other words, the system lost an average of $18,800 per patient for the care of a hip fracture. These costs would represent the potential savings to the health care system of an effective FLS program based on how many of these hip fractures would be prevented for a 2-year period.
Expected reduction in hip re-fractures based on our FLS effectiveness
In the first part of this prospective study, we enrolled 200 patients equally into two groups to determined effectiveness of our FLS program. At 4 months from the fracture, the rate of patients on recommended treatment was determined for each group. The rate of treatment for the letter group at 4 months was only 6% compared to the intervention group with 77% [8]. We used this enrollment rate for the purpose of calculations in this cost analysis for effectiveness of our FLS program.
Medication costs
The cost of medications had the greatest impact on cost of FLS care. The Israel Ministry of Health is mandated by law to require the health insurance to pay for most of the costs of medications including bisphosphonates, but coverage also includes the more expensive options such as teriparatide or denosumab after a hip fracture. For other types of fragility-related fractures, such as wrist or spine, the more expensive options are not covered unless approved by an endocrinologist. Therefore, the calculations and cost-benefit analysis determined here would be different if the initial fracture was not a hip fracture or if we used a different algorithm for choosing medications (more in the Discussion section) (Supplementary Material 2, www.currentsurgery.org).
Most patients were started on either zoledronic acid or denosumab. There were some patients that were started on teriparatide and two other medications (available upon request). The costs of medications were calculated for each patient then averaged for the group (available upon request).
Expected savings from FLS program
Cost-benefit ratio from the perspective of the hospital compared to the health insurance
The greatest difference in cost of the FLS program between the hospital compared to the insurance is that only the health insurance bears the added expense of the medications. The FLS management costs are the same for each. The potential savings or “benefit” for the health insurance is greater since the costs for treating fractures is more (Supplementary Material 3, www.currentsurgery.org).
Cost-benefit ratio for a hospital-based FLS program
The FLS we started was orthopedic-inspired and based in the hospital. Using the non-medication FLS cost of $172 and expected per patient savings and for reducing future fractures of $30, we calculated an overall projected loss per patient of $142 over 2 years. Since medications were not a factor for the hospital, this loss did not affect the hospital cost-benefit ratio.
Cost-benefit ratio for a health insurance-based FLS program
Here we assume that the FLS would be managed by the insurance provider. Using the average per patient FLS cost (over 2 years) of $1,250 (that includes medication) and savings of $579 (for reduced cost of treating secondary fractures), we calculate an overall loss of $671 per patient per 2 years. It is important to note that costs calculated here include the FLS program, the DEXA scans and the medications. The medications were the bulk of the cost at $1,078 (86% of the costs).
If patients had all been given zoledronic acid, then the FLS medication costs would have been reduced to $628 and total costs reduced to $800 giving a loss of $221 per patient for 2 years. If generic oral bisphosphonates (alendronate once weekly) had been given, the medication cost would have been $298 or a total cost of $470 giving a profit for the FLS program of $109 per patient for 2 years.
| Discussion | ▴Top |
In this study, we prospectively analyzed the costs and savings of an FLS program. We learned that the most critical determinant was medication costs which depend on the treatment algorithm. If we had used oral bisphosphonates instead of the more expensive and presumably effective options, then the FLS program would have saved $109 per patient over 2 years.
When this study was started, it was assumed that the amount saved would be more than enough to pay for the program. After all, hip fracture treatment is expensive, and the salary of a nurse coordinator is relatively low. Our initial back-of-the-napkin analysis estimated the cost of a 1/2 time nurse coordinator (for our volume of hip fractures) at $30,000 per year, and with the hospital loss of $4,132 for each hip fracture, it would take reducing only 7.3 hip fractures per year for a hospital-based FLS program to pay for itself. In our institution, we treat over 600 hip fractures per year and with only 1% reduction in secondary fractures, having six less hip fractures is possible. For the national insurance, starting an FLS program is even more favorable. With loss of $14,670 per hip fracture, it would take reducing only one hip fractures per year to pay the part-time coordinator salary. Indeed, Majumdar et al [9] used exactly this type of analysis and concluded that their FLS program only costs $50 per patient. They assumed an FLS coordinator salary of $33.3/h with 30% overhead and we used $34/h and 20% overhead. However, the actual costs of an FLS program are more complex.
Although the beneficial effects of osteoporosis medication are likely to reduce the rate of re-fractures for up to 5 years [10], we calculated the savings based on only the first 2 years. This would have the effect of under-estimating the benefit of an FLS program. In addition, the cost analysis here does not consider the added expense and morbidity of patients who because of a secondary fracture never return to a functional state. Up to 80% of patients after a hip fracture never return to their pre-injury level and 27% require nursing home care [11]. Finally, this cost analysis does not consider the number of lives saved by reducing re-fractures.
Overall savings
Cooper et al [12] used a Markov model in the Canadian system using different assumptions to calculate an average savings of $88 per patient over 10 years (with range from -$379 to $693 (ratio of 1 AUD = 0.76 USD). Their FLS used bisphosphonates for post-fracture treatment. Solomon et al [13] did a cost-effectiveness analysis of an FLS program after a hip fracture in the US health care system using a Markov computer simulation model. They concluded that an FLS program would reduce hip fractures by 1.1% which is comparable to the 1.11% used in this study. They showed a cost savings of their post-fracture FLS of $6.68 per patient. Their model used fracture reduction rates using bisphosphonate therapy from previously published data.
McLellan et al [14] did an elegant cost-effectiveness analysis using 8 years of data from the West Glasgow FLS. For a hypothetic cohort of 1000 fragility fracture patients, they estimated a reduction of 11 secondary hip fractures (1.1%) and an additional 7 (0.7%) non-hip fractures saving £21,000 or approximately $28.2 per patient enrolled. They used bisphosphonates in 55% of the patients and Ca/Vit D only in 40%.
Major et al [15] did what they term was a “micro-costing” approach trying to determine costs and effectiveness of an Australian FLS. Adjusting their 3-year estimates for our 2-year interval, their FLS program cost $229 per patient compared to our estimate of $172. Using their simulation for 5% treatment level for non-FLS care, they found a reduction of 62 fractures per 1,000 patients over a 3-year period and an overall FLS cost savings of $411 per patient (adjusted to 2-year savings). They assumed medication cost of only $62 for a 2-year treatment. Their estimate was generated using data from a pharmaceutical website and not measured directly in their patient cohort. This is in stark contrast to our actual cost of $1,078 based on medications given for the patient cohort. If the medication cost in this study had been only $62 for the same period, the FLS cost savings in this study would have been $349 (insurance savings) comparable to their results of $411. This emphasizes one of the primary conclusions of this study, that medication choice appears to be the key factor on whether the FLS program ultimately saves or loses money for the system.
Our study strengths include real-life data rather than primarily estimates. For example, in determining cost of hip fracture care, the cost for each patient was individually calculated that included factors such as length of stay and complications. The actual effectiveness of our program was determined prospectively and perhaps most important, the medication costs were based on actual patient data.
Our study had several limitations. We did not include patients with moderate or severe dementia. This was because the study requires consent, and the intervention program required the patient to be in contact monthly. Another limitation of the study is that our system recognizes complications related to the initial procedure only up to 30 days. If the patient was re-admitted after that time, but was still related to the index procedure, it would not have been counted and therefore costs might be underestimated. The 2-year horizon used for this analysis was chosen since the Horizon study [6] had the best data for fracture reduction. This study was not designed to prospectively follow individual patients. In addition, medication costs over a longer time frame would presumably be more effective at reducing secondary fractures but would also significantly increase costs. We believe that the conclusions of this study would remain unchanged that medication costs are the critical factor in profitability of an FLS program.
The greatest argument might be that our medication algorithm was not appropriate. Patients with a higher risk of re-fracture based on their combined FRAX scores and DEXA scans might benefit from the more effective and expensive options whereas most of the patients may only need bisphosphonates [11]. Before starting the study, we met with the endocrinologists that advise each of the four different national health insurance programs in Israel and all agreed with the proposed algorithm. Importantly, all the medications are part of the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for osteoporosis treatment [16]. The Israel Ministry of Health has approved all the medications for treatment after hip fractures given the unique risk in this group of patients.
We believe that the analysis here is not unique to the Israel health care system. The components of the analysis and the relative costs of the components should be comparable within other health care systems.
We hope the information provided in this analysis can help the interested physician advocate or insurance provider design an effective and cost-efficient FLS for an increasingly elderly population. Everyone loses with low energy hip fractures: patients, hospitals and the national health care system. The solution is to invest properly in a well-designed FLS program. More study is needed in this important area.
| Supplementary Material | ▴Top |
Suppl 1. Costs and reimbursements for hospital versus insurance for non-hip and non-vertebral fractures.
Suppl 2. Cost of medications* - Health Insurance Costs (2018) - over 2 years (USD).
Suppl 3. Average savings per patient (the benefit of an FLS system) for hospital versus health insurance for reduction in secondary fractures, adjusted for cohort of 83 patients (same as intervention group) - in USD.
Acknowledgments
None to declare.
Financial Disclosure
No direct funding was received. A small contribution to the research unit was received by Amgen Co.
Conflict of Interest
Gershon Zinger, Amit Davidson, Noa Sylvetsky, Yekin Levy and Amos Peyser declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants in the study.
Author Contributions
GZ planned the prospective study, analyzed the results, and wrote the manuscript. AD planned the cost analysis and entered all the cost data. NS was the endocrinology consultant including providing the medication protocol. YL and AP assisted with manuscript preparation and revision.
Data Availability
The authors declare that data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Edwards BJ, Koval K, Bunta AD, Genuario K, Hahr A, Andruszyn L, Williams M. Addressing secondary prevention of osteoporosis in fracture care: follow-up to "own the bone". J Bone Joint Surg Am. 2011;93(15):e87.
doi pubmed - Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721-739.
doi pubmed - Center JR. Fracture burden: what two and a half decades of Dubbo osteoporosis epidemiology study data reveal about clinical outcomes of osteoporosis. Curr Osteoporos Rep. 2017;15(2):88-95.
doi pubmed - Bunta AD, Edwards BJ, Macaulay WB, Jr., Jeray KJ, Tosi LL, Jones CB, Sietsema DL, et al. Own the Bone, a System-Based Intervention, Improves Osteoporosis Care After Fragility Fractures. J Bone Joint Surg Am. 2016;98(24):e109.
doi pubmed - Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, McLellan AR, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22(7):2051-2065.
doi pubmed - Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
doi pubmed - Zinger G, Sylvetsky N, Levy Y, Steinberg K, Bregman A, Yudkevich G, Peyser A. Efficacy of orthopaedic-inspired osteoporosis management: a secondary fracture prevention program after a fracture of the hip in a prospective randomized study. OTA Int. 2021;4(2):e122.
doi pubmed - Zinger G, Sylvetsky N, Levy Y, Steinberg K, Bregman A, Yudkevich G, Peyser A. Early benefits of a secondary fracture prevention programme. Hip Int. 2021.
doi pubmed - Majumdar SR, Lier DA, McAlister FA, Johnson JA, Rowe BH, Beaupre LA. Cost-effectiveness of osteoporosis interventions to improve quality of care after upper extremity fracture: results from a randomized trial (C-STOP Trial). J Bone Miner Res. 2019;34(7):1220-1228.
doi pubmed - Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int. 2011;22(Suppl 3):457-460.
doi pubmed - Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103(2A):12S-17S; discussion 17S-19S.
doi pubmed - Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the Concord Minimal Trauma Fracture Liaison service, a prospective, controlled fracture prevention study. Osteoporos Int. 2012;23(1):97-107.
doi pubmed - Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res. 2014;29(7):1667-1674.
doi pubmed - McLellan AR, Wolowacz SE, Zimovetz EA, Beard SM, Lock S, McCrink L, Adekunle F, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011;22(7):2083-2098.
doi pubmed - Major G, Ling R, Searles A, Niddrie F, Kelly A, Holliday E, Attia J, et al. The costs of confronting osteoporosis: cost study of an Australian fracture liaison service. JBMR Plus. 2019;3(1):56-63.
doi pubmed - Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, et al. Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(4):1-42.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
