| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Short Communication
Volume 13, Number 1, September 2023, pages 6-11
The Effects of Cisplatin on Gastrostomy Site Healing
Jacqueline Renee Bootha, f, Sahar Emami Naeinib, c, f, g, Hesam Khodadadid, Evila Lopes Sallesb, Thalyta Xavier de Medeirose, Austin DeLaneyd, Edward Jim Krused, e, Achuta Kumar Guddatid, e, Babak Babanb, James Kenneth Byrdd, e, g
aRousso Adams Facial Plastic Surgery, Birmingham, AL, USA
bDental College of Georgia, Augusta University, Augusta, GA 30912, USA
cDepartment of Oral Biology and Diagnostic Sciences, Augusta University, Augusta, GA 30912, USA
dMedical College of Georgia, Augusta University, Augusta, GA, USA
eGeorgia Cancer Center, Augusta University, Augusta, GA, USA
fThese authors contributed equally to the article.
gCorresponding Author: James Kenneth Byrd, Medical College of Georgia, Augusta University, Augusta, GA, USA; Sahar Emami Naeini, Department of Oral Biology and Diagnostic Sciences, Augusta University, Augusta, GA 30912, USA
Manuscript submitted December 1, 2022, accepted January 16, 2023, published online April 16, 2023
Short title: Cisplatin and Gastrostomy Site Healing
doi: https://doi.org/10.14740/jcs468
| Abstract | ▴Top |
Background: Gastrostomy tube placement is sometimes necessary during chemoradiation for head and neck cancer (HNC), but it is associated with worse swallowing outcomes. Despite best efforts, the need for gastrostomy cannot be perfectly predicted, and some patients develop the need for gastrostomy while undergoing chemotherapy. To date, the impact of cisplatin on gastrostomy site healing has not been investigated. The aim of this study is to compare the levels of procollagen, connective tissue growth factor (CTGF), and CD26 in the gastrostomy sites of mice at varying timepoints after cisplatin versus saline.
Methods: For this study we used 32 C57BL/6 mice. Cisplatin (1 mg/kg) was injected intraperitoneally into the mice from treatment groups. Control groups received the same volume of normal saline intraperitoneally. Mice underwent gastrostomy tube placement at 7, 10, 14, and 17 days post cisplatin or saline therapy. Ten days after gastrostomy placement, mice were sacrificed, and the gastrostomy site tissue was examined. We measured the levels of procollagen type 1, CTGF, and CD26 by flow cytometry. Masson’s trichrome stain was used for qualitative comparison of collagen formation in surgical site tissue.
Results: Masson’s trichrome staining showed more collagen formation in surgical site tissue at 17 versus 7 days post-cisplatin. Levels of procollagen type 1 by flow cytometry were significantly higher in the control group compared to the treatment group (P < 0.0001) for each time point. There was a statistically significant difference in procollagen type 1 between the 7 days post-chemotherapy group and the 17 days post-chemotherapy group (P = 0.0024). The percentage of cells with procollagen, CTGF, and CD26 in the 7 days post-saline control group were similar to the percentage in the 14 days post-cisplatin treatment group.
Conclusions: CTGF, CD26, and procollagen were decreased by cisplatin in gastrostomy site tissue at each time point, but levels 14 days after a cisplatin treatment approximate the levels 7 days after saline. These results suggest that it is safe to proceed with gastrostomy tube placement 14 - 17 days after cisplatin because the CTGF, CD26, and procollagen levels approximate the levels 7 days after saline. Additional studies are needed to extrapolate to humans.
Keywords: Cisplatin; Healing; Gastrostomy site; CTGF; Procollagen
| Introduction | ▴Top |
Worldwide, head and neck cancer (HNC) accounts around 6% of new cancer diagnoses [1]. The annual incidence in the United States is estimated at 15 per 100,000 with over 12,000 deaths attributable to the disease [2]. Based on the surveillance, epidemiology and end results (SEER) query of patients from 1989 to 2014, over 50% of patients with HNC will eventually undergo cancer-directed surgery [3]. Radiotherapy also continues to play a significant role and is offered to nearly 75% of HNC patients as a single agent or in combination therapy, based on a report by Ratko et al [4]. The platinum-based chemotherapy agent cisplatin is used in over 50% of cases as part of chemoradiation therapy (CRT) regimens [5].
In patients with HNC, feeding difficulties often arise as a result of disease process or treatment. The combined chemotherapy and radiation treatments that patients with HNC receive often cause oropharyngeal mucositis, odynophagia, and swallowing dysfunction. This is particularly true when chemotherapy is a component of the regimen since it is known to heighten the effect of radiation. However, high-dose cisplatin remains a mainstay for HNC treatment since no alternatives have been able to achieve superior, or even equivalent results [6]. Therefore, many of these patients will require gastrostomy tubes for supplemental nutrition [7, 8]. In a study by Locher et al [8], 35% of patients with HNC underwent gastrostomy tube placement.
Stiernberg et al [9] reported that cisplatin affects wound healing by decreasing wound strength. Pivotally, Engelmann et al [10] showed that preoperative cisplatin administration in rats led to reduced connective tissue proliferation, inhibition of fibroblasts and endothelial cells, and hindrance of vessel proliferation. Taken in conjunction, cisplatin has an impact on the healing of gastrostomy sites. Improper adhesion of the stomach to the abdominal wall may lead to peristomal leakage, leakage into the abdomen and subsequent infection necessitating exploratory laparotomy [11]. Considering this, the timing of gastrostomy tubes is often debated in the management of HNC patients.
While the effect of prophylactic gastrostomy placement on patient survival and tumor control is unknown, benefit was assumed since one series that found weight loss was the strongest independent predictor of survival [12]. This led some clinicians to advocate for all CRT patients to receive prophylactic gastrostomy tube [13]. However, the late toxicities of prophylactic gastrostomy tube placement, specifically, long-term gastrotomy tube dependence and esophageal stricture, impose lifelong tolls that are unfortunately common [12, 14, 15]. Limiting gastrostomy tube use is believed to be beneficial for long-term swallowing outcomes.
On the other hand, some patients who were initially presumed capable of oral intake develop the need for additional supplementation. When this case arises, gastrostomy tubes are placed after initiation of treatment. While reactive gastrostomy tube placement while on chemotherapy is utilized, there are little data on healing of the surgical site. Anecdotal data have shown poor healing in HNC patients that underwent gastrostomy tube placement during treatment with CRT [16]. In vitro and in vivo data demonstrate that cisplatin negatively impacts wound healing and production of collagen [9, 16, 17]. When clinicians wait until there is undeniable need for a gastrostomy tube, the question remains: when is the optimal timing for gastrostomy placement for patients receiving cisplatin to minimize the negative impact on wound healing?
Currently, there is no consensus regarding the timing of gastrostomy tube placement in HNC patients undergoing chemotherapy. There is no existing evidence to guide decision-making and many institutions have adopted prophylactic gastrostomy tube placement practices. This may potentially result in poor long-term swallowing outcomes and unnecessary gastrostomy tube placement, as well as unnecessary associated complications. Insight into the timing for safe gastrostomy placement in cisplatin therapy patients could help guide practices that adopt reactive gastrostomy placement.
Wound healing is a complex process that depend on several cellular and molecular components like collage and connective tissue growth factor (CTGF). Collagen is a main component in all phases of wound healing. It supports new blood vessel and granulation tissue formation [18]. The cells responsible for the majority production of collagen are the fibroblasts. According to the literature, fibroblasts CD26+ are responsible for the production of the most part of the components of the collagen fiber [19]. Besides the collagen production, during the wound healing, cellular proliferation and differentiation process are very important. CTGF is a matricellular protein that induce proliferation and differentiation of the cells in the wound side helping on the tissue repair [20].
The study aims to determine that time point by comparing wounds between mice treated with intraperitoneal saline versus cisplatin. Mice will undergo gastrostomy tube placement at 7, 10, 14, or 17 days after chemotherapy.
Regarding to important role of these three markers in wound healing process we hypothesize that healing gastrostomy wound levels of procollagen, CD26, and CTGF in cisplatin treated mice will be decreased compared to the wounds of controls 7 days after chemotherapy. We expect tissue levels of these markers to approach that of controls when the gastrostomy is performed at timepoints further from a dose of cisplatin therapy. The incremental increase in the delay of surgery after completion of cisplatin therapy is designed to determine when gastrostomy could be performed while maintaining reasonable wound healing function.
| Materials and Methods | ▴Top |
Sixteen ICR treatment mice were administered 1 mg/kg cisplatin by intraperitoneal route. The mice were divided into four groups with four mice in each, undergoing gastrostomy on post-chemotherapy days 7, 10, 14, or 17. Sixteen ICR control mice with four mice in each group underwent saline infusion rather than cisplatin infusion, followed by gastrostomy tube placement at the same time points for comparison. Gastrostomy placement was performed with the following technique (Fig. 1). Mice were anesthetized using 3% isoflurane. A left flank incision was made to place the tube through. A mid-abdominal incision was made to access the gastric body. Anchoring sutures were placed to lift the anterior gastric wall. The gastric wall is then incised with a microscalpel. The tube was placed into the stomach and pulled through from a tunnel under the skin and fixed to the lateral abdominal wall with a 2/0 silk suture. Skin and muscle layers were closed using 4/0 Vicryl suture.
 Click for large image | Figure 1. Gastrostomy tube insertion. The figure represents the technique that the gastrostomy placement was performed. Left flank incision was made (a) to place the tube through (b). The tube was placed into stomach and fixed to the lateral abdominal wall (c). |
All mice were sacrificed 10 days after placement of the gastrostomy tube and the tissue surrounding the site was harvested. Formalin-fixed paraffin-embedded gastrostomy site tissues were sectioned and stained with Masson’s trichrome for qualitative collagen analysis. Samples from the gastrostomy sites were also prepared for flow cytometry analysis comparing levels of CTGF, procollagen type 1, and CD26.
The Institutional Review Board approval is not applicable. The study was conducted in compliance with the Animal Study: 2011-0062.
| Results | ▴Top |
Masson’s trichrome staining shows scant collagen formation in surgical site tissue from mice that had surgery 7 days post-chemotherapy (Fig. 2). In contrast, the surgical site tissue from mice that were 17 days post-chemotherapy had collagen similar to a mouse sacrificed after gastrostomy placement alone. Levels of procollagen type 1 were significantly higher in the control group compared to the treatment group with P < 0.0001 for each time point. When comparing the treatment groups, there was a statistically significant difference in procollagen type 1 between the 7 days post-cisplatin group and the 17 days post-cisplatin group (Fig. 3a). The percentage of cells positive for procollagen in the 7 days post-saline control group was similar to the percentage in the 14 days post-cisplatin treatment group (P = 0.0024) (Fig. 3b). The same was true of the levels of cells positive for both CTGF and CD26; levels at 14 days post-cisplatin were comparable to levels at 7 days post-saline (Fig. 3c).
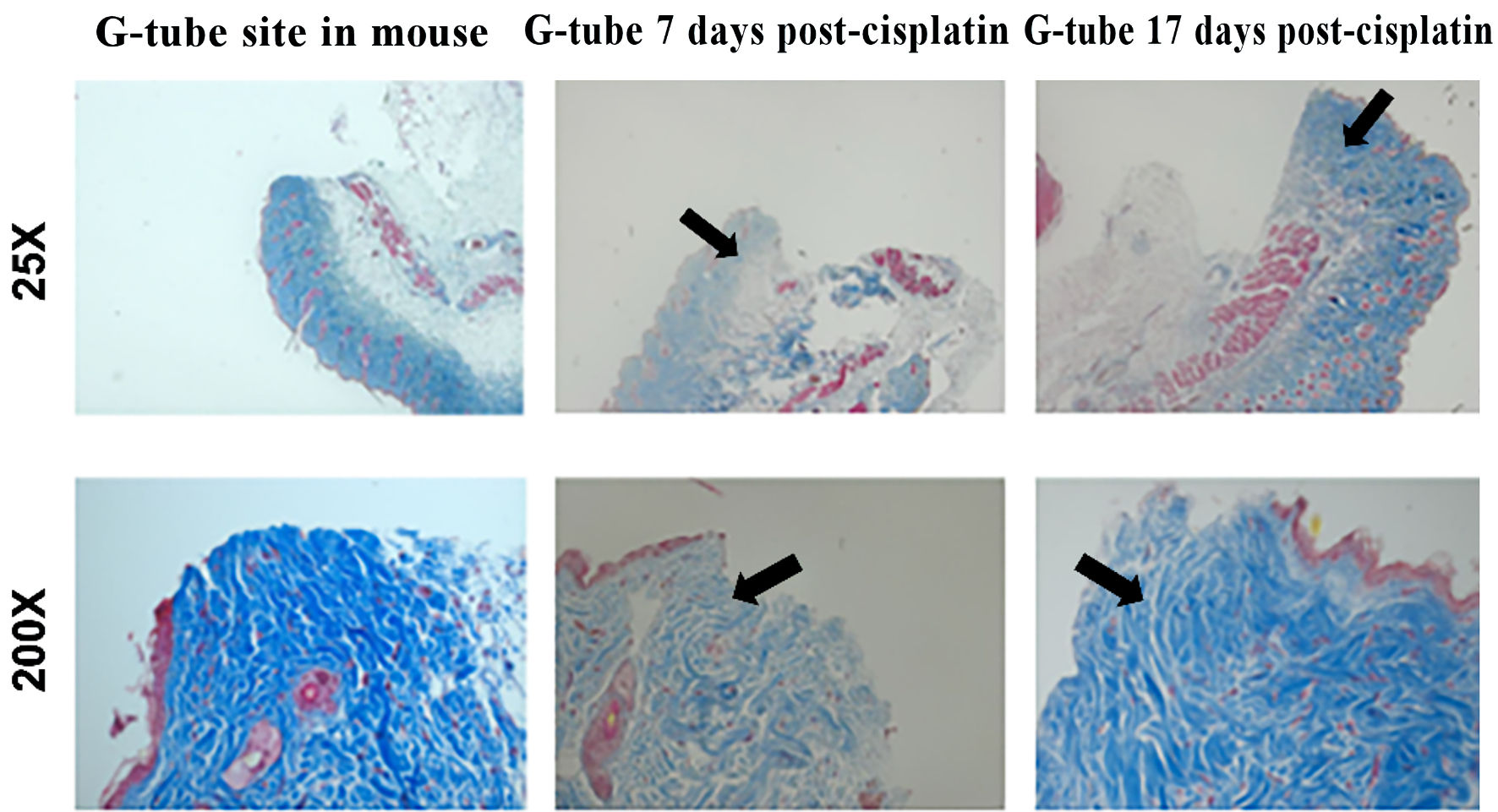 Click for large image | Figure 2. Masson’s trichrome staining showed more collagen formation in surgical site tissue at 17 versus 7 days post-cisplatin. The arrows heads show the collagen fibers. |
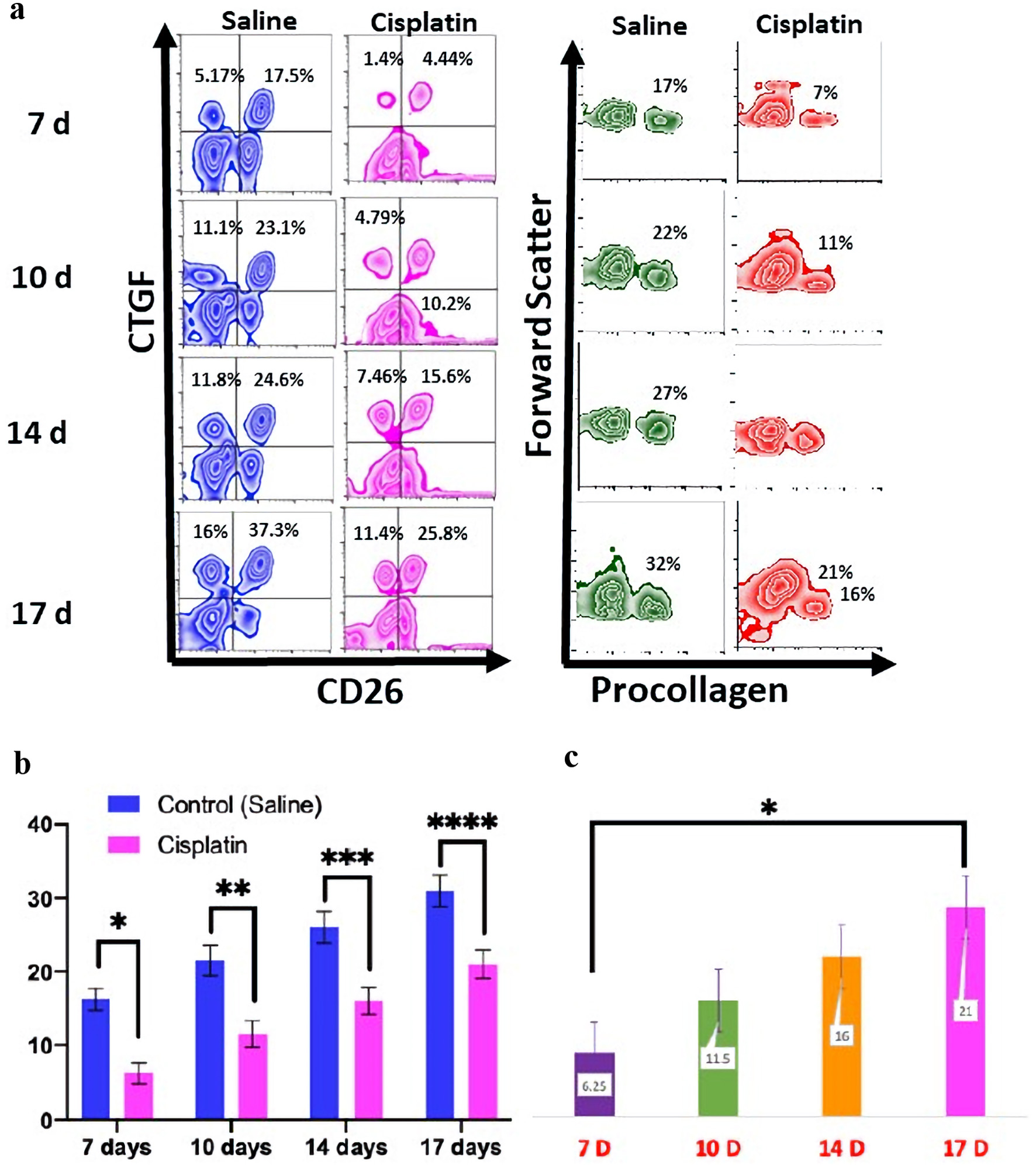 Click for large image | Figure 3. The flow cytometry for the CTGF, CD26, and procollagen expression from day 7 to 17. Levels at 14 days post-cisplatin were comparable to levels at 7 days post-saline (a). The statistical analyses were performed using two-way ANOVA with Tukey multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. The graph shows the levels of procollagen type 1 by flow cytometry were a significant higher expression that was present in the control group compared to the treatment group (P < 0.0001) for each time point (b). There was a statistically difference in procollagen type 1 between the 7 days post-chemotherapy group and the 17 days post-chemotherapy group for each time point (c). CTGF: connective tissue growth factor; ANOVA: analysis of variance. |
| Discussion | ▴Top |
Many patients with HNC develop difficulties with their nutritional status throughout the course of their treatment. The commonly used regimens of chemotherapy and radiation often lead to odynophagia, swallowing dysfunction, and mucositis. When these side effects become excessive and oral feeding is not tolerable, routes of enteral feeding are pursued. The main routes used are nasogastric tubes (NGTs) and gastrostomies. Although the insertion of a NGT is generally a comparatively less complicated procedure, there are risks. Mucosal erosion, discomfort, perforations, pulmonary injury, and aspiration are all possible complications [21]. In addition, NGTs are not a long-term solution for patients who need continued nutritional support. The use of NGT for longer than 6 weeks portends an increased risk of serious adverse events [21]. Therefore, gastrostomy tubes are commonly used to provide nutritional support long-term via direct access to the lumen of the stomach. However, this route requires a more involved and costly procedure. In addition, a longer healing time is required before the gastrostomy site can be accessed and utilized for feeding. Poor wound healing and adhesion of the gastrostomy site to the abdominal wall can lead to morbid complications including septicemia, peritonitis, hemorrhage, pneumoperitoneum, and dislodging of the gastrostomy tube [11].
There is therefore additional concern about complications of gastrostomy when the patient is undergoing chemotherapy when the need for a gastrostomy tube arises. Cisplatin is a commonly used, platinum-based alkylating chemotherapy agent. This agent is known to impact wound healing by reducing fibroblasts, inhibiting angiogenesis, and reducing connective tissue proliferation [10]. As such, debate remains regarding the optimal timing of gastrostomy creation when cisplatin-based chemotherapy has been initiated.
The chronology of the interaction between cisplatin and the healing wound was examined in this study. The results of this study suggest that it may be safe to proceed with gastrostomy tube placement 14 - 17 days after cisplatin because the CTGF, CD26, and procollagen type 1 levels in this timeframe approximate the levels 7 days after saline. Furthermore, the levels of type 1 procollagen 17 days after cisplatin are significantly improved compared to levels 7 days after cisplatin. This new data can help guide providers and patients on the timing of post-cisplatin gastrostomy tube placement as they carefully weigh the risks and benefits of individual cases.
There are several notable limitations to this study. First, it is a preliminary animal study, rather than a human study. Whether intraperitoneal cisplatin in a mouse behaves the same as an infusion in a human is unknown. Additionally, the percutaneous gastrostomy technique commonly used in humans for minimally invasive tube placement was not possible in the mouse mode. Finally, although appropriate microscopic markers of wound healing in cisplatin-treated mice were similar to control mice at days 14 -17, the tensile strength of the surgical sites was not tested.
As expected, healing was impacted by cisplatin with significantly less CTGF, CD26, and procollagen at each time point when compared to saline. The results show that the levels 14 days after a cisplatin treatment approximate the levels 7 days after saline. Additionally, procollagen levels are significantly higher in the surgical site when performed 17 days after cisplatin when compared to 7 days after cisplatin. This was confirmed qualitatively with the Masson’s trichrome stain. Thus, careful patient-oriented consideration of goals and timing for nutritional support should be undertaken with the multidisciplinary team. Additional studies are needed to directly extrapolate to humans.
Acknowledgments
Our thanks to the Augusta University Research Institute Intramural Grant Committee who made this study possible.
Financial Disclosure
This project was supported by internal grant program funding awarded to Dr. James Kenneth Byrd by office of vice president for research at Augusta University ((706) 721-6100). The resources for other parts of the current manuscript were provided by seed money from Dental College of Georgia, Augusta University awarded to BB.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: JRB, SEN, BB and JKB. Methodology: HK, ELS, and TXM. Data analysis: SEN, AD, EJK, and AKG. Writing: JRB, SEN, and HK. Manuscript review: BB, JKB, and ELS.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sexton GP, Walsh P, Moriarty F, O'Neill JP. The changing face of Irish head and neck cancer epidemiology: 20 years of data. Eur Arch Otorhinolaryngol. 2022;279(6):3079-3088.
doi pubmed pmc - Rettig EM, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 2015;24(3):379-396.
doi pubmed - Crippen MM, Elias ML, Weisberger JS, Brady JS, Eloy JA, Baredes S, Park RCW. Refusal of cancer-directed surgery in head and neck squamous cell carcinoma patients. Laryngoscope. 2019;129(6):1368-1373.
doi pubmed - Ratko TA, Douglas GW, de Souza JA, Belinson SE, Aronson N. In: Radiotherapy Treatments for Head and Neck Cancer Update. Rockville (MD). 2014.
- Xiang M, Holsinger FC, Colevas AD, Chen MM, Le QT, Beadle BM. Survival of patients with head and neck cancer treated with definitive radiotherapy and concurrent cisplatin or concurrent cetuximab: A Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2018;124(23):4486-4494.
doi pubmed - Siu LL, Waldron JN, Chen BE, Winquist E, Wright JR, Nabid A, Hay JH, et al. Effect of standard radiotherapy with cisplatin vs accelerated radiotherapy with panitumumab in locoregionally advanced squamous cell head and neck carcinoma: a randomized clinical trial. JAMA Oncol. 2017;3(2):220-226.
doi pubmed - Ahmed KA, Samant S, Vieira F. Gastrostomy tubes in patients with advanced head and neck cancer. Laryngoscope. 2005;115(1):44-47.
doi pubmed - Locher JL, Bonner JA, Carroll WR, Caudell JJ, Kilgore ML, Ritchie CS, Roth DL, et al. Gastrostomy tube placement and use in patients with head and neck cancer. Head Neck. 2012;34(3):422-428.
doi pubmed - Stiernberg CM, Williams RM, Hokanson JA. Influence of cisplatin on wound healing—an experimental model. Otolaryngol Head Neck Surg. 1986;95(2):210-212.
doi pubmed - Engelmann U, Krug J, Sonntag W, Jacobi GH. [The effect of cisplatin on the healing of intestinal anastomosis in the rat. Microangiography and light microscopy studies]. Urol Int. 1984;39(2):73-79.
doi pubmed - Complications following surgery of the septum and turbinates, in complications in otolaryngology. Head and Neck Surgery. 2013. Georg Thieme Verlag KG: Stuttgart.
- Locher JL, Bonner JA, Carroll WR, Caudell JJ, Keith JN, Kilgore ML, Ritchie CS, et al. Prophylactic percutaneous endoscopic gastrostomy tube placement in treatment of head and neck cancer: a comprehensive review and call for evidence-based medicine. JPEN J Parenter Enteral Nutr. 2011;35(3):365-374.
doi pubmed - Nguyen NP, North D, Smith HJ, Dutta S, Alfieri A, Karlsson U, Lee H, et al. Safety and effectiveness of prophylactic gastrostomy tubes for head and neck cancer patients undergoing chemoradiation. Surg Oncol. 2006;15(4):199-203.
doi pubmed - Bhayani MK, Hutcheson KA, Barringer DA, Lisec A, Alvarez CP, Roberts DB, Lai SY, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck. 2013;35(11):1634-1640.
doi pubmed - Chen AM, Li BQ, Lau DH, Farwell DG, Luu Q, Stuart K, Newman K, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78(4):1026-1032.
doi pubmed - Garsed K, Armstrong R, Scott BB. Delayed healing of percutaneous endoscopic gastrostomy site during chemotherapy. Gut. 2007;56(7):1028-1029.
doi pubmed pmc - Hendricks T, Martens MF, Huyben CM, Wobbes T. Inhibition of basal and TGF beta-induced fibroblast collagen synthesis by antineoplastic agents. Implications for wound healing. Br J Cancer. 1993;67(3):545-550.
doi pubmed pmc - Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering (Basel). 2021;8(5):63.
doi pubmed pmc - Worthen CA, Cui Y, Orringer JS, Johnson TM, Voorhees JJ, Fisher GJ. CD26 identifies a subpopulation of fibroblasts that produce the majority of collagen during wound healing in human skin. J Invest Dermatol. 2020;140(12):2515-2524.e2513.
doi pubmed pmc - Alfaro MP, Deskins DL, Wallus M, DasGupta J, Davidson JM, Nanney LB, M AG, et al. A physiological role for connective tissue growth factor in early wound healing. Lab Invest. 2013;93(1):81-95.
doi pubmed pmc - Prestwich RJD, Murray LJ, Williams GF, Tease E, Taylor L, George C, Cardale K, et al. Impact of choice of feeding tubes on long-term swallow function following chemoradiotherapy for oropharyngeal carcinoma. Acta Oncol. 2019;58(8):1187-1196.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
